产品号 #100-0276_C
cGMP,稳定无饲养层维持培养基,适用于人ES和iPS 细胞
cGMP,稳定无饲养层维持培养基,适用于人ES和iPS 细胞
Innovation is a core value at STEMCELL. The evolution to mTeSR™ Plus reflects that and our desire to serve the next generation of pluripotent stem cell researchers.

这款稳定的无饲养层维持培养基适用于人多能干细胞 (hPSC),可以在保持细胞质量的同时,让您享受无需周末换液的的灵活日程安排并提升细胞生长特性。mTeSR™ Plus 遵循相关的 cGMP 生产,确保提供最高质量和一致性,适用于基础研究、细胞治疗及新药研究等应用。它基于mTeSR™1(目录号 #85850),这是目前最广泛使用的 hPSC 无饲养层细胞培养基。凭借包括 FGF2 在内的稳定的关键培养基成分和增强的 pH 缓冲功能,您可以使用 mTeSR™ Plus 来维持细胞质量属性,并通过每日或限制性换液来提高细胞扩增速度。每批 mTeSR™ Plus 5X 补充剂均用于配制完整的 mTeSR™ Plus 培养基,然后在使用人多能干细胞 (hPSC) 的培养试验中进行性能测试。
mTeSR™ Plus 与多种培养基质兼容,包括 Corning® Matrigel® hESC 认证基质和Vitronectin XF™(目录号:07180,由 Nucleus Biologics 开发和生产)。
如需获取更多质量信息,请访问www.stemcell.com/compliance。
如需申请 mTeSR™ Plus 的 FDA 主文件授权书 (LOA),请点击此处。
亚型
专用培养基
细胞类型
多能干细胞
种属
人
应用
细胞培养,扩增,培养
品牌
TeSR
研究领域
疾病建模,药物发现和毒理检测,干细胞生物学
制剂类别
无血清
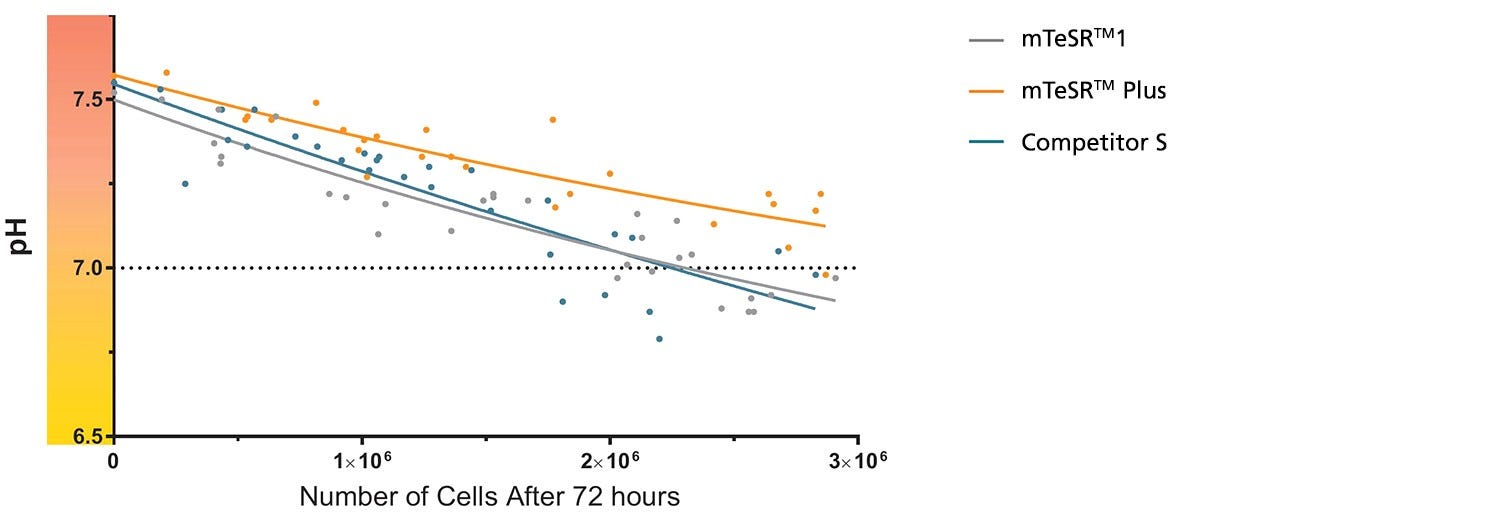
Figure 1. mTeSR™ Plus Maintains Optimal pH Levels Throughout a Weekend-Free Protocol
The pH of spent medium from hPSCs cultured in mTeSR™ Plus is higher than that of hPSCs cultured in mTeSR™1 and other flexible-feeding medium at similar cell densities. pH and cell numbers were measured after a 72-hour period without feeding. Range of cell numbers shown represent different densities that would be observed throughout a typical passage. This demonstrates that feeds can be skipped for two days at any time during routine maintenance using mTeSR™ Plus while maintaining a pH above 7.0. Note: Cultures were fed double the standard medium volume prior to the 72-hour period without feeds in all media and cell numbers are from one well of a 6-well plate.
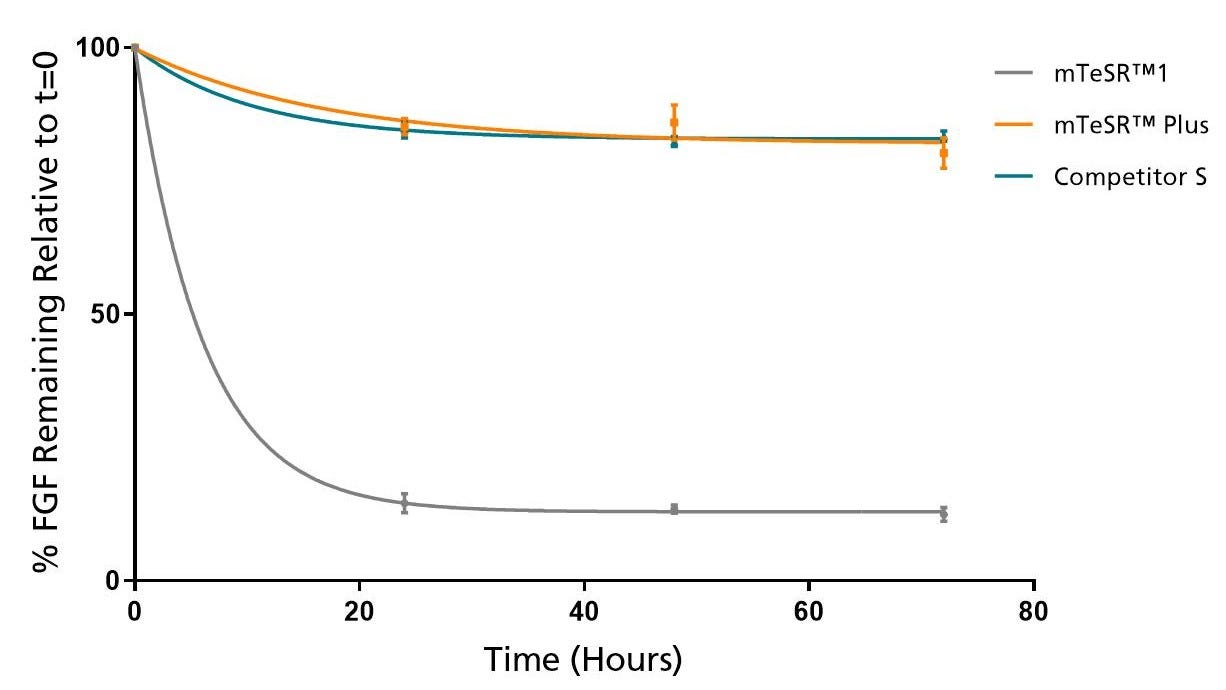
Figure 2. mTeSR™ Plus Maintains Consistent Levels of FGF2 Throughout a Weekend-Free Protocol
FGF2 levels remain high in mTeSR™ Plus when cultured at 37°C over a 72 hour time period. Measured by ELISA.
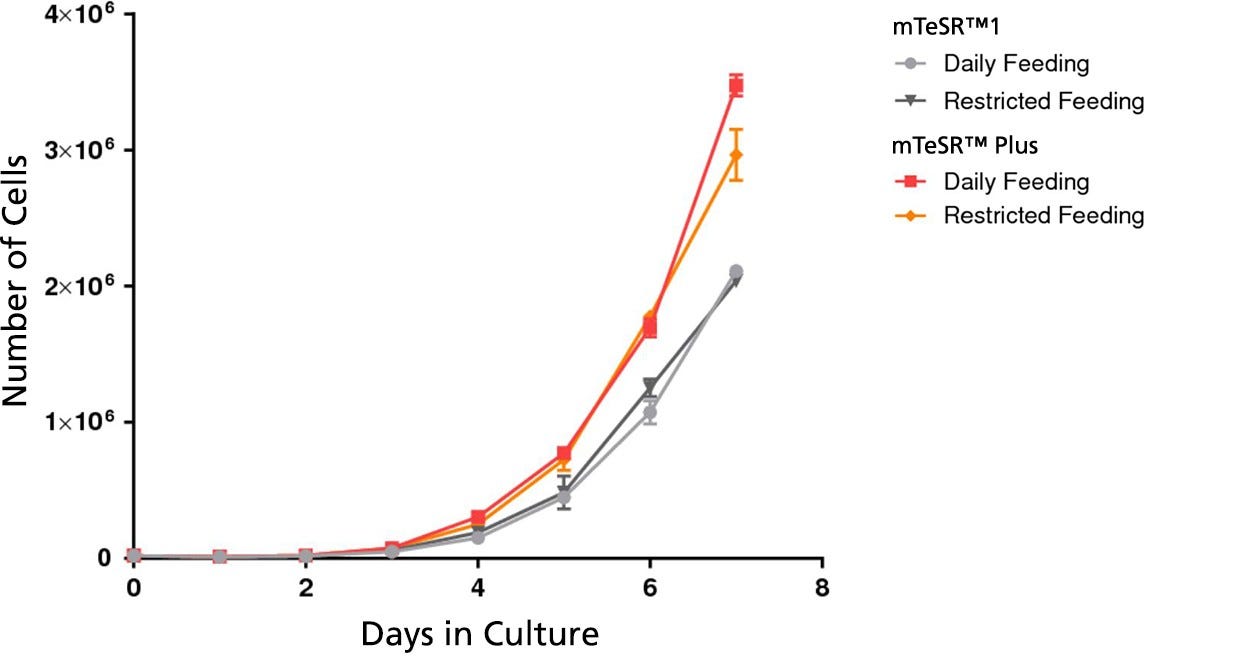
Figure 3. mTeSR™ Plus Supports Higher Cell Numbers
Growth curves were obtained for human ES (H9) cells cultured in mTeSR™1 or mTeSR™ Plus on Corning® Matrigel® matrix over 7 days with either daily feeds or restricted feeds. Growth curves were determined by seeding 20,000 cells per well of a 6-well plate as aggregates and counting the cell numbers each day in duplicate wells.
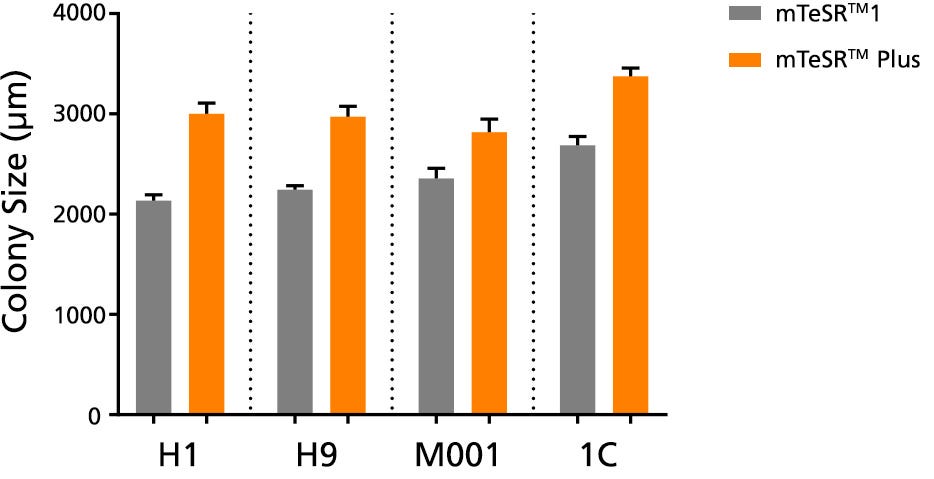
Figure 4. Larger Colonies are Observed in mTeSR™ Plus Cultures
The average colony size per passage (± SEM) was obtained for human ES (H1, H9) and iPS (STiPS-M001, WLS-1C) cells cultured in mTeSR™1 (daily feeds) or mTeSR™ Plus (restricted feeds) on Corning® Matrigel® over 10 passages. Size was determined by measuring representative colony diameters at harvest. Note that this data is representative of cultures passaged at a 7-day passaging interval; smaller colony size should be expected if using shorter passaging intervals.
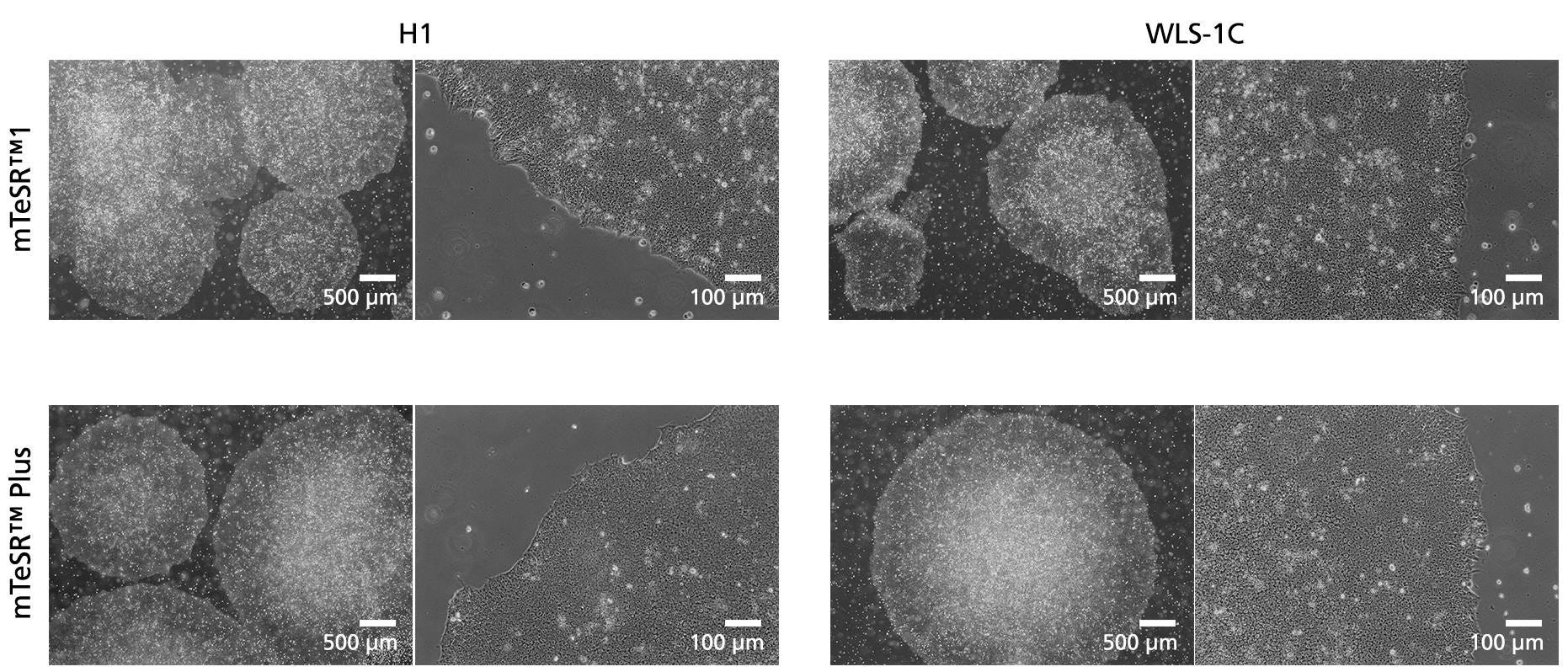
Figure 5. Normal human ES and iPS Cell Morphology is Observed in mTeSR™ Plus Cultures
Images depict undifferentiated human hES (H1) and iPS (WLS-1C) cells cultured on Corning®️ Matrigel®️ matrix in mTeSR™1 with daily feeds or mTeSR™ Plus with restricted feeds. Cells retain the prominent nucleoli and high nuclear-to-cytoplasmic ratio characteristic of this cell type after 10 passages. Densely packed cells and multi-layering are prominent when cells are ready to be passaged.
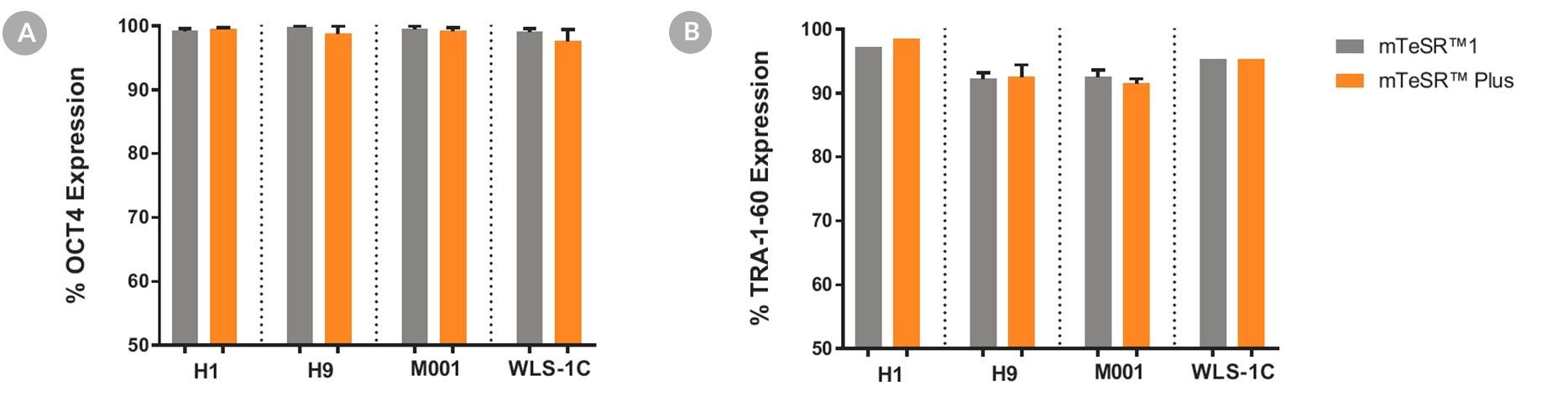
Figure 6. Cells Cultured in mTeSR™ Plus Medium with Restricted Feeding Express Undifferentiated Cell Markers
Human ES (H1, H9) and iPS (WLS-1C, STiPS-M001) cells were characterized using flow cytometry for undifferentiated cell markers, (A) OCT3/4 and (B) TRA-1-60. Graphs show average expression (± SEM) results from analyses of duplicate wells every 5 passages for up to 10-15 passages in mTeSR™1 (daily feeds), or mTeSR™ Plus (restricted feeds).
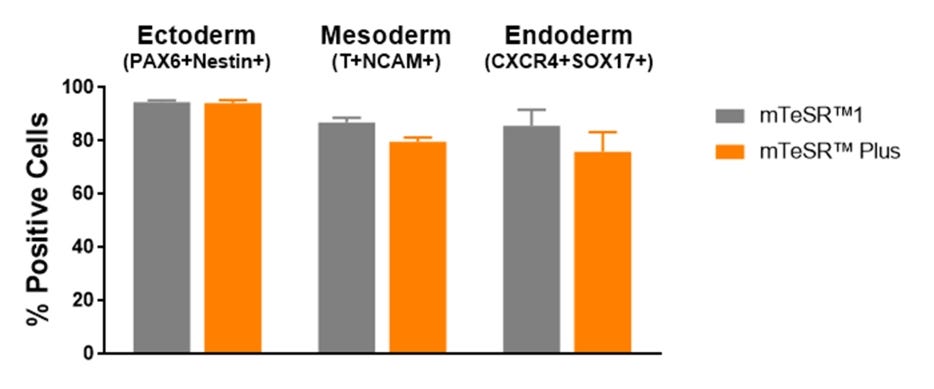
Figure 7. Cells Maintained in mTeSR™ Plus with Restricted Feeding Have Comparable Differentiation Efficiencies to Cells Maintained in mTeSR™1
Human ES (H1, H9) and iPS (WLS-1C, STiPS-M001) cells were maintained in mTeSR™1 (daily feeds) or mTeSR™ Plus (restricted feeds). Cells were differentiated using directed differentiation protocols and subjected to flow cytometry analysis. Graphs show average expression (± SEM) results from the 4 cell lines. The markers used for flow cytometry for each germ layer are listed in the bar titles.
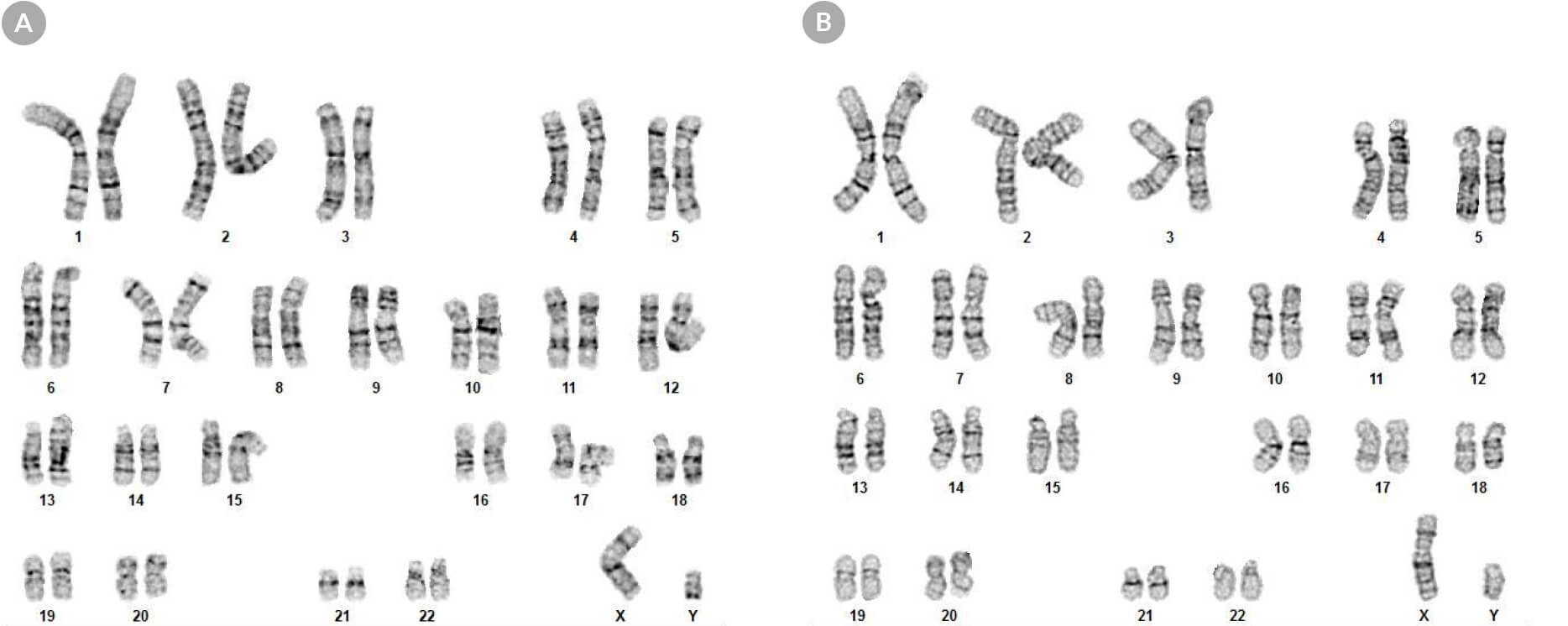
Figure 8. hPSCs Cultured in mTeSR™ Plus with Restricted Feeding Maintain a Normal Karyotype
Karyograms of (A) human ES (H1) and (B) iPS (WLS-1C) cells cultured in mTeSR™ Plus for 30 passages shows a normal karyotype is retained.
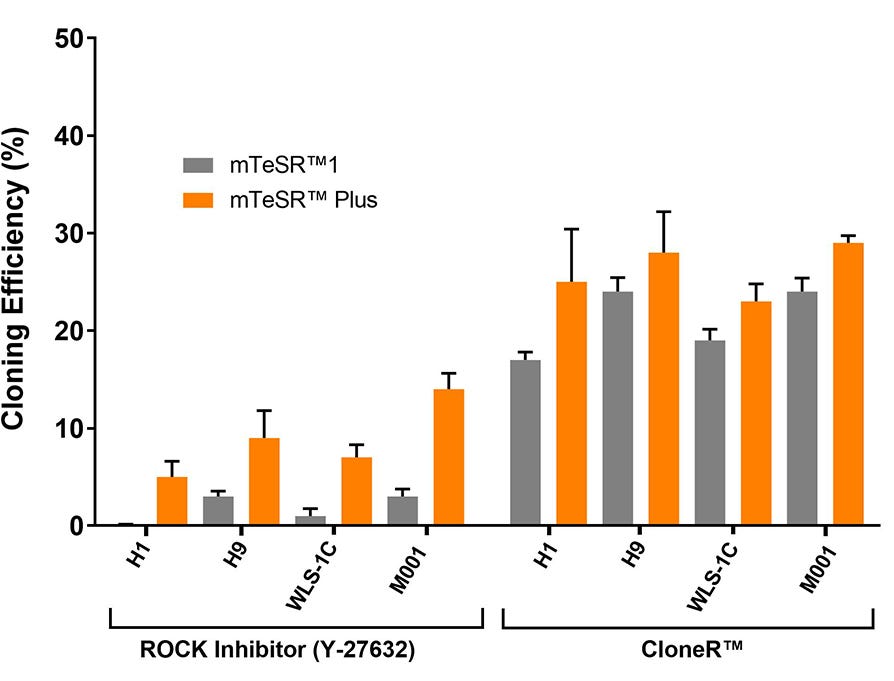
Figure 9. High Cloning Efficiency of hPSCs in mTeSR™ Plus Supplemented with CloneR™
hPSCs (H1, H9, WLS-1C, and STiPS-M001) plated in mTeSR™ Plus with CloneR™ demonstrate cloning efficiencies equal to or greater than hPSCs in mTeSR™1 with CloneR™. Cells were seeded at clonal density (25 cells/cm²) in mTeSR™1 or mTeSR™ Plus on CellAdhere™ Vitronectin™ XF™-coated plates. n ≧ 3 biological replicates.
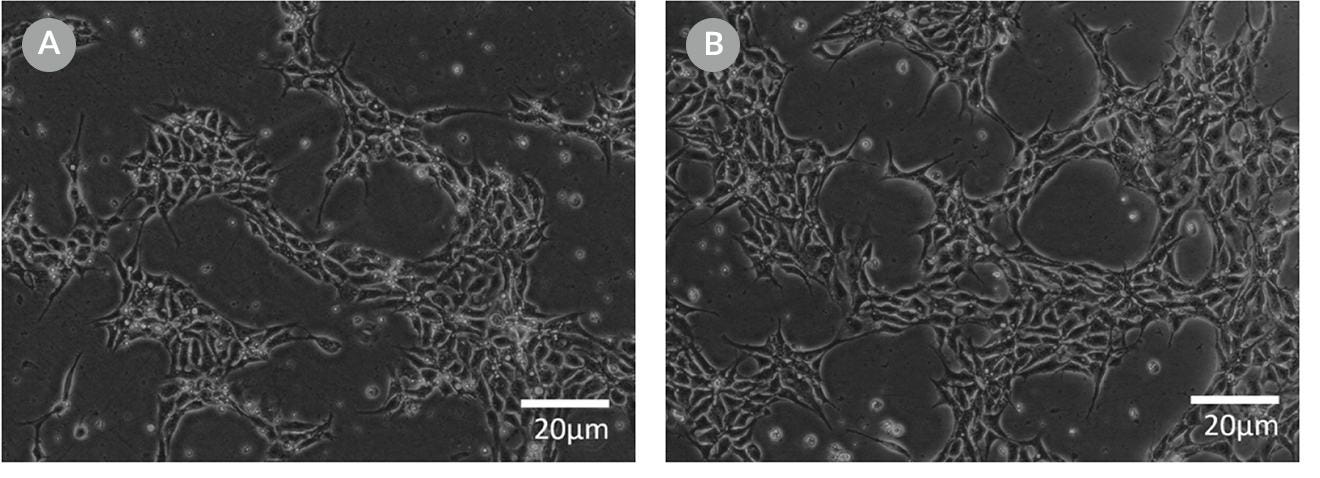
Figure 10. Representative Cell Morphology 24 Hours After RNP Electroporation in mTeSR™1 and mTeSR™ Plus
H1-eGFP ES cells were plated in (A) mTeSR™1 and (B) mTeSR™ Plus and supplemented with CloneR™ immediately following RNP electroporation. Images were taken 24 hours after electroporation.
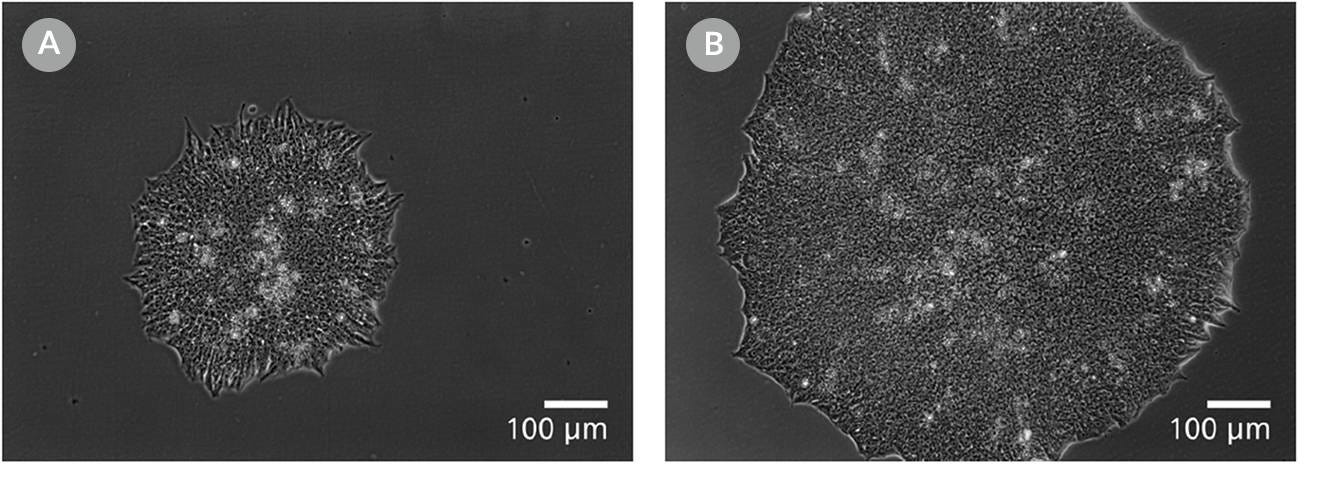
Figure 11. Clones Derived in mTeSR™ Plus are Larger and Ready to Be Picked at an Earlier Timepoint
Representative images of human ES (H9) colonies taken 8 days following singlecell plating at clonal density (25 cells/cm²) in either (A) mTeSR™1 or (B) mTeSR™ Plus supplemented with CloneR™ on CellAdhere™ Vitronectin™ XF™-coated plates.
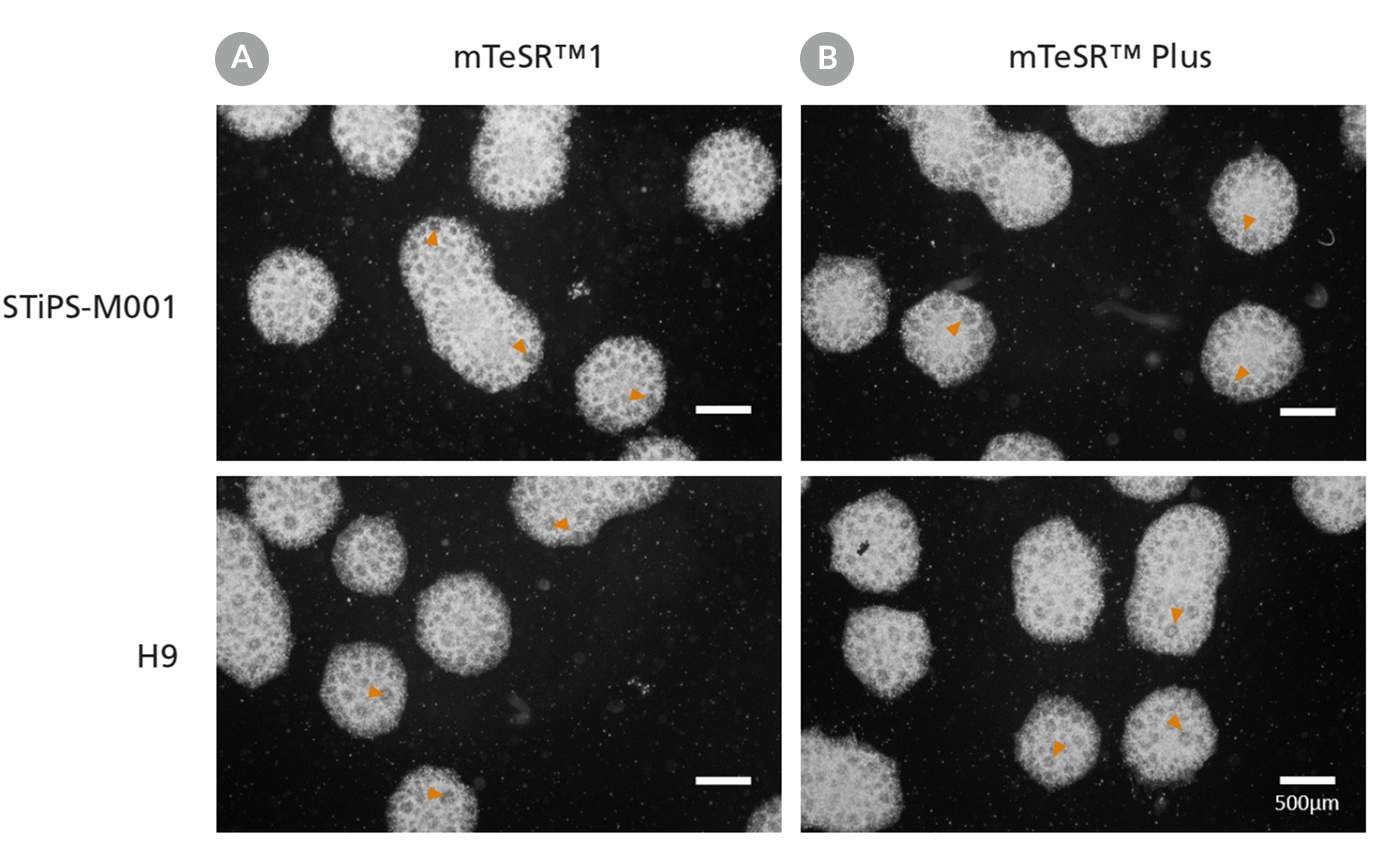
Figure 12. Generation of Neural Progenitor Cells from hPSCs Maintained in mTeSR™ Plus
Human ES (H9) and iPS (STiPS-M001) cells were maintained in (A) mTeSR™1 with daily feeds or (B) mTeSR™ Plus with restricted feeds and differentiated using an embryoid body (EB)-based protocol with STEMdiff™ SMADi Neural Induction Kit. Neural progenitor cells derived from hPSCs maintained in either mTeSR™1 or mTeSR™ Plus clearly display neural rosettes (arrowheads) after replating EBs.
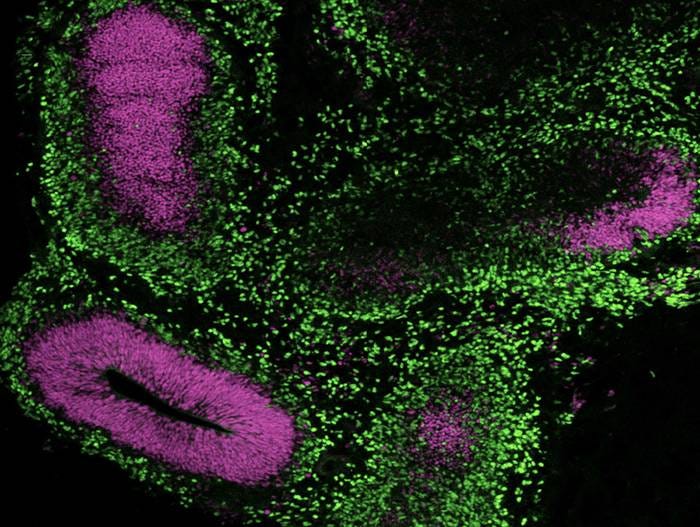
Figure 13. Generation of Cerebral Organoids from hPSCs Maintained in mTeSR™ Plus
Human ES (H9) cells were cultured with mTeSR™ Plus and directed to cerebral organoids using the STEMdiff™ Cerebral Organoid Kit. Image shows apical progenitor marker SOX2 (purple) and neuronal marker TBR1 (green).
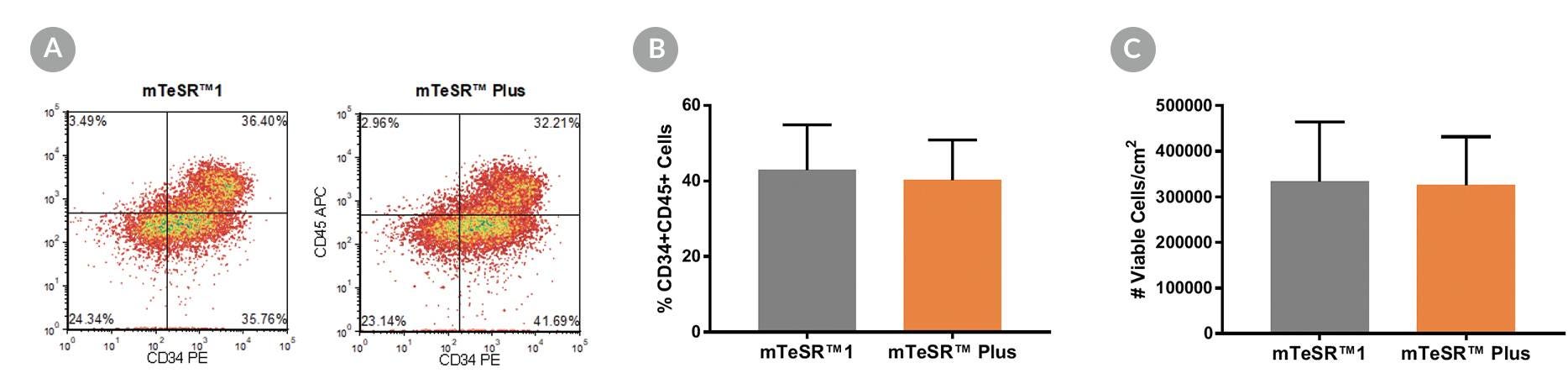
Figure 14. Generation of Hematopoietic Progenitor Cells from hPSCs Maintained in mTeSR™ Plus
Human ES (H1, H9) and iPS (STiPS-M001, WLS-1C) cell lines maintained in mTeSR™1 (daily feeds) or mTeSR™ Plus (restricted feeds) were differentiated to hematopoietic progenitor cells using the STEMdiff™ Hematopoietic Kit. At the end of the differentiation period, cells were harvested from the supernatant and analyzed by flow cytometry for co-expression of CD34+ and CD45+ . (A) Representative density plots showing CD34+ and CD45+ expression, (B) percentage of cells co-expressing CD34+ and CD45+ , and (C) total number of viable cells harvested are shown. Data are expressed as the mean (± SEM); n=4.
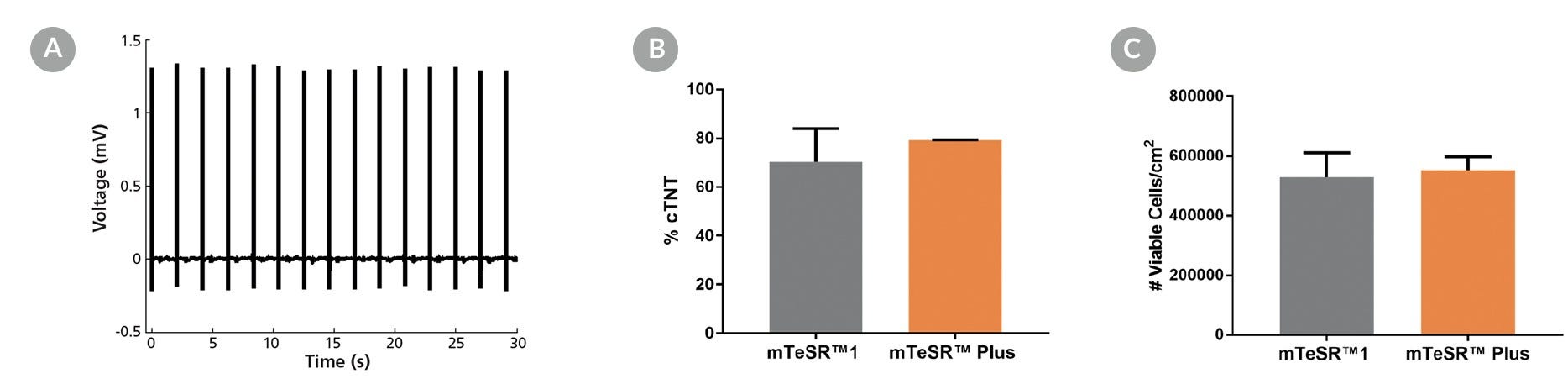
Figure 15. Generation of Cardiomyocytes from hPSCs Maintained in mTeSR™ Plus
Human ES (H9) and iPS (WLS-1C) cells were maintained in mTeSR™1 (daily feeds) or mTeSR™ Plus (restricted feeds) and differentiated to cardiomyocytes using the STEMdiff™ Cardiomyocyte Differentiation Kit. At the end of the differentiation period, cells were harvested and analyzed by microelectrode array (MEA) and flow cytometry. (A) Representative MEA voltage recordings of cardiomyocytes (day 20) demonstrate a characteristic electrical profile and stable beat rate. (B) Percentages of cells expressing cTNT and (C) total number of viable cells harvested are shown. Data are expressed as the mean (± SEM); n=2.
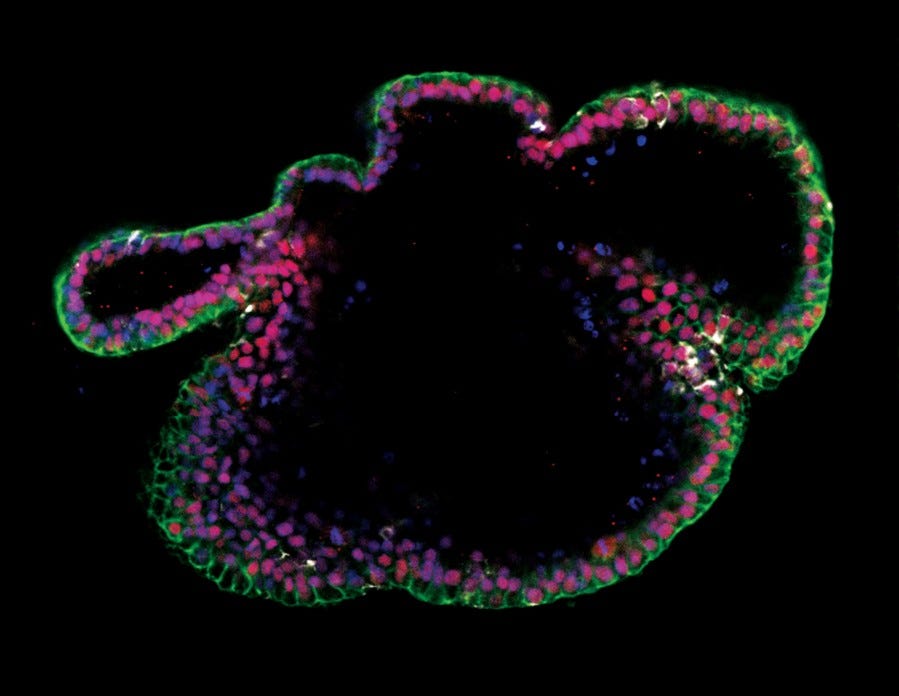
Figure 16. Generation of Intestinal Organoids from hPSCs Maintained in mTeSR™ Plus
Human ES (H9) cells were cultured with mTeSR™ Plus and directed to intestinal organoids using the STEMdiff™ Intestinal Organoid Kit. Image shows markers of the intestinal epithelium EpCAM (green) and CDX2 (red), and intestinal mesenchyme marker vimentin (white). Nuclei are counterstained with DAPI (blue).
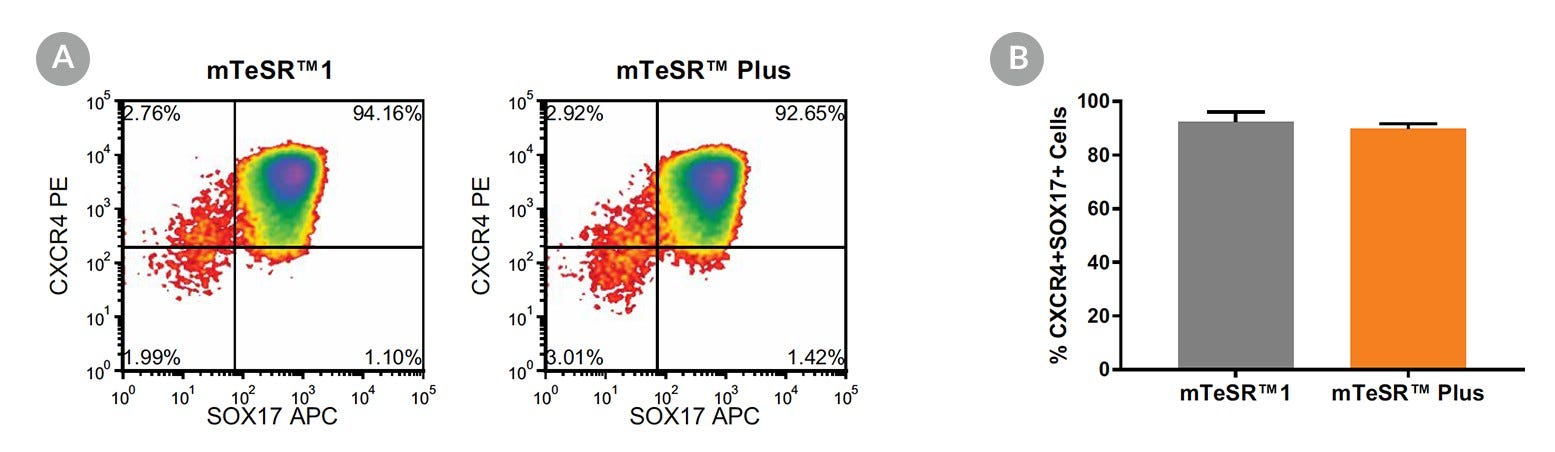
Figure 17. Generation of Definitive Endoderm from hPSCs Maintained in mTeSR™ Plus
(A) Representative density plots showing CXCR4 and SOX17 expression in cells cultured in mTeSR™1 (daily feeds) or mTeSR™ Plus (restricted feeds), following 5 days of differentiation using the STEMdiff™ Definitive Endoderm Kit. (B) Quantitative analysis of definitive endoderm formation in multiple hPSC lines (H9, STiPS-M001, WLS-1C) maintained with mTeSR™1 or mTeSR™ Plus as measured by co-expression of CXCR4 and SOX17. Data are expressed as the mean percentage of cells (± SEM) expressing both markers; n=3.
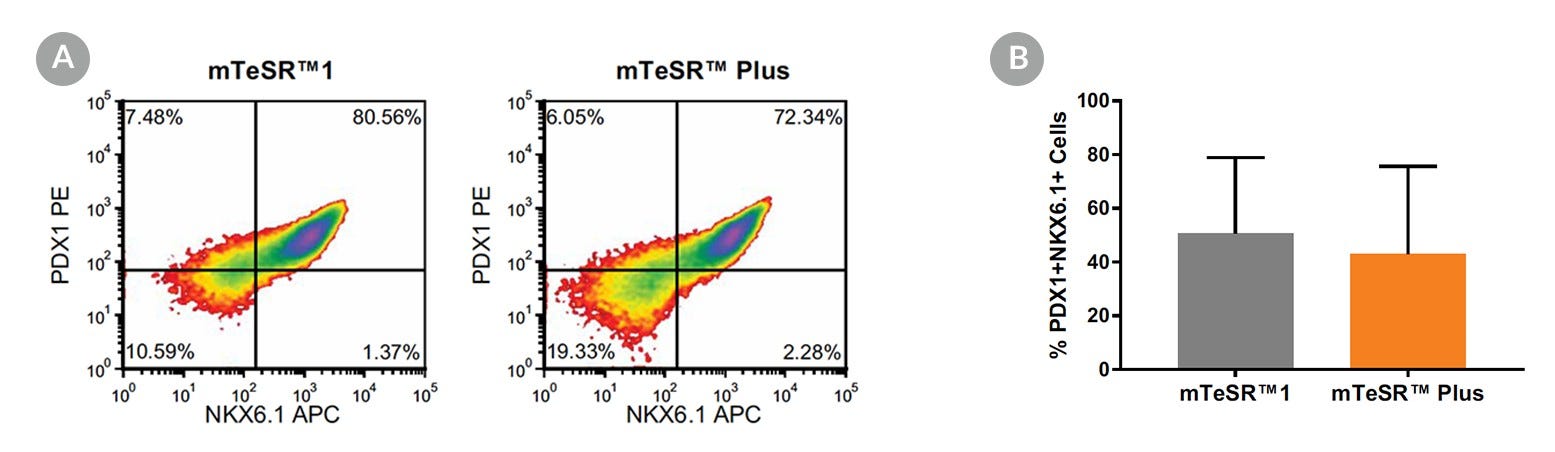
Figure 18. Generation of Pancreatic Progenitors from hPSCs Maintained in mTeSR™ Plus
(A) Representative density plots showing PDX-1 and NKX6.1 expression in cells cultured in mTeSR™1 (daily feeds) or mTeSR™ Plus (restricted feeds), following differentiation using the STEMdiff™ Pancreatic Progenitor Kit. (B) Quantitative analysis of pancreatic progenitor formation in multiple hPS (H9, STiPS-M001, WLS-1C) cell lines maintained with mTeSR™1 or mTeSR™ Plus as measured by co-expression of PDX-1 and NKX6.1. Data are expressed as the mean percentage of cells (± SEM) expressing both markers; n=3.
Find supporting information and directions for use in the Product Information Sheet or explore additional protocols below.
This product is designed for use in the following research area(s) as part of the highlighted workflow stage(s). Explore these workflows to learn more about the other products we offer to support each research area.
Thank you for your interest in IntestiCult™ Organoid Growth Medium (Human). Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration
| Species | Human |
|---|---|
| Formulation Category | Serum-Free |
cGMP级、无酶的人多能干细胞选择与传代试剂

用于提高人胚胎干细胞和有道多能干细胞在单细胞工作流程中的存活率添加物

一次性使用、免维持的人诱导多能干细胞,冷冻

人多能干细胞系,冷冻
扫描二维码或搜索微信号STEMCELLTech,即可关注我们的微信平台,第一时间接收丰富的技术资源和最新的活动信息。
如您有任何问题,欢迎发消息给STEMCELLTech微信公众平台,或与我们通过电话/邮件联系:400 885 9050 INFO.CN@STEMCELL.COM。