产品号 #100-2078_C
无血清和无BPE培养基,用于将人原代呼吸道上皮细胞分化为成熟的包含基底细胞、纤毛细胞和分泌细胞的顶端外翻呼吸道类器官
无血清和无BPE培养基,用于将人原代呼吸道上皮细胞分化为成熟的包含基底细胞、纤毛细胞和分泌细胞的顶端外翻呼吸道类器官
Rinsing solution for cultureware to prevent cell adhesion
Cell culture supplement

Cell culture supplement

Microwell culture plates for easy and reproducible production of embryoid bodies and spheroids
Compatible antibodies for purity assessment of isolated cells
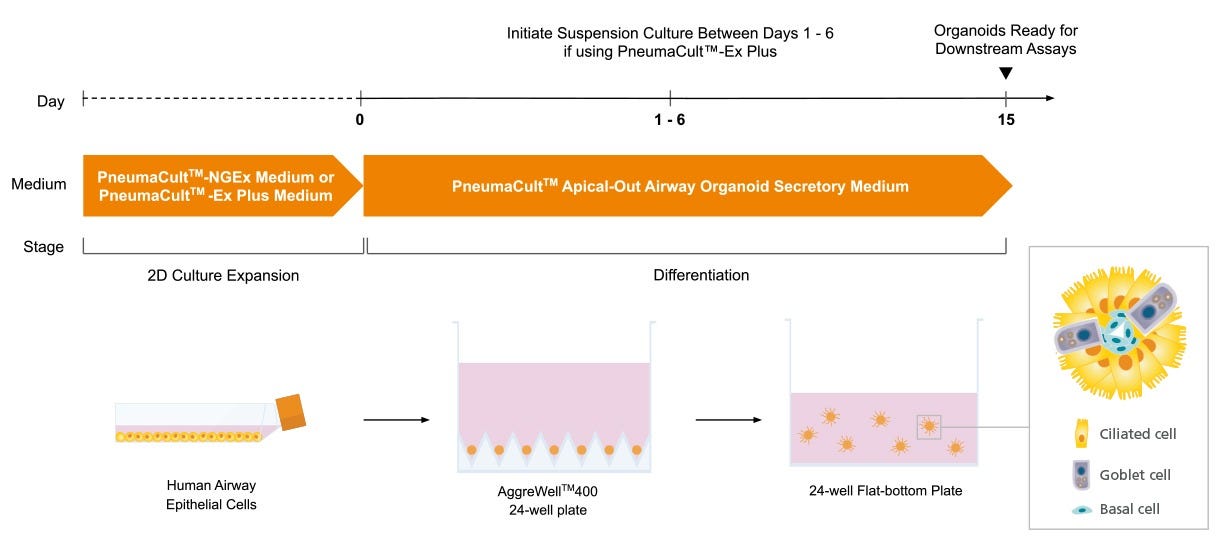
Figure 1. Overview of the PneumaCult™ Apical-Out Airway Organoid Secretory Medium Workflow
Prior to generating apical-out airway organoids, human airway epithelial cells (HAECs) are expanded using PneumaCult™-NGEx (Catalog #100-1505) or PneumaCult™-Ex Plus (Catalog #05040) Medium in a two-dimensional expansion phase. The cells are then plated into an AggreWell™400 24-well plate (Catalog #34450) and allowed to aggregate and differentiate using PneumaCult™ Apical-Out Airway Organoid Secretory Medium. For cells expanded in PneumaCult™-NGEx, aggregates are cultured in the AggreWell™400 plate for 15 days, after which, fully differentiated organoids are transferred to suspension culture and are immediately assay-ready. When using cells expanded in PneumaCult™-Ex Plus, aggregates are transitioned to a suspension culture after 1 - 6 days in the AggreWell™400 plate. The optimal transition time is determined by donor-specific characteristics. Regardless of the expansion medium used, apical-out airway organoids are ready for downstream applications by Day 15.
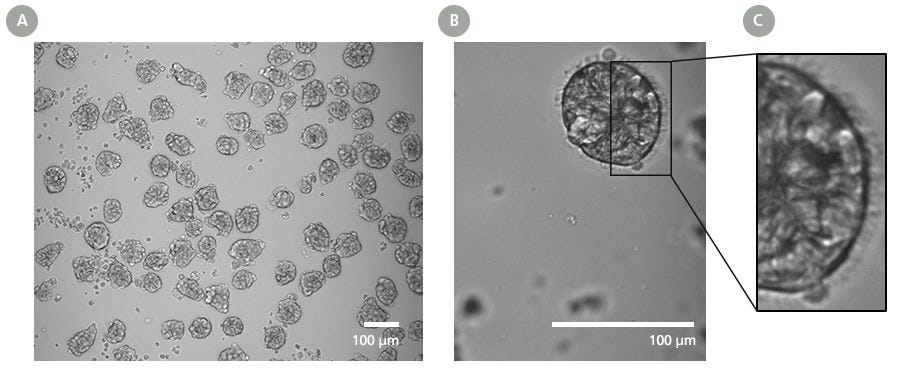
Figure 2. PneumaCult™ Apical-Out Airway Organoid Secretory Medium Enables Generation of Mature Organoids with Distinct Outward-Facing Ciliated Morphology
(A) Day 15 organoids generated using PneumaCult™ Apical-Out Airway Organoid Secretory Medium were imaged and appear uniformly shaped, demonstrating the consistency of the differentiation process.
(B) Mature organoids display a cell-dense core containing basal cells and (C) an epithelium that presents ciliated cells on the outer surface, indicating their successful apical-out polarity. This consistent morphology found in mature apical-out airway organoids supports reliable performance in downstream assays and enhances reproducibility across respiratory research applications. All organoids shown were derived from passage 4 human airway epithelial cells.
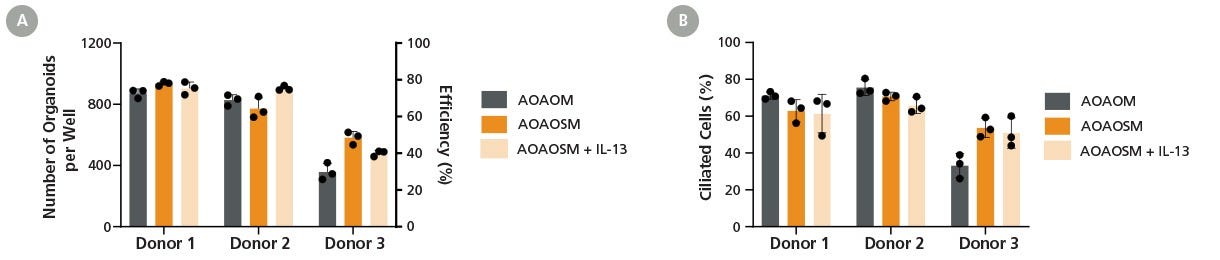
Figure 3. PneumaCult™ Apical-Out Airway Organoid Secretory Medium Supports Efficient Organoid Formation and Robust Ciliogenesis Across Donors
Apical-out airway organoids were generated by seeding passage 4 human bronchial epithelial cells (HBECs) derived from 3 donors into three culture conditions: PneumaCult™ Apical-Out Airway Organoid Medium (AOAOM; Catalog #100-0620), PneumaCult™ Apical-Out Airway Organoid Secretory Medium (AOAOSM), and PneumaCult™ AOAOSM supplemented with 5 ng/mL IL-13 (AOAOSM + IL-13). IL-13 (Catalog #78029) was included to stimulate immunoregulatory cytokine-regulated goblet cell hyperplasia.
(A) The number of organoids formed per well in a AggreWell™400 24-well plate on Day 15. Data points represent mean ± SD taken from at least three independent wells of a 24-well plate. Efficiency is expressed as the percentage of apical-out airway organoids at the end of culture relative to the total number of aggregates used to seed the cultures.
(B) Ciliated cell percentage of organoids generated in each culture condition from three donors. Organoid-forming efficiency and ciliated cell percentage remained stable across all culture conditions for Donors 1 and 2, with a slight decrease observed for Donor 3, indicating the intrinsic donor-to-donor variability. Addition of IL-13 did not impact organoid yield or ciliated cell differentiation capacity. These results demonstrate that PneumaCult™ AOAOSM supports robust and reproducible organoid generation and ciliogenesis across varying experimental conditions, enabling healthy and disease-relevant airway phenotype modeling.
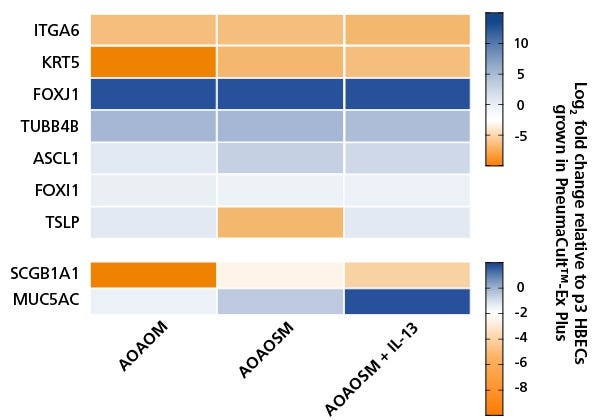
Figure 4. PneumaCult™ Apical-Out Airway Organoid Secretory Medium Increases Expression of Secretory Cell Markers and Is Further Enhanced by Addition of IL-13
Heatmap showing fold changes in the RNA levels of common differentiation markers in apical-out airway organoids cultured in PneumaCult™ Apical-Out Airway Organoid Medium (AOAOM; Catalog #100-0620), PneumaCult™ Apical-Out Airway Organoid Secretory Medium (AOAOSM) and PneumaCult™ Apical-Out Airway Organoid Secretory Medium supplemented with 5 ng/mL IL-13 (AOAOSM + IL-13). Results were normalized against human bronchial epithelial cells (HBECs) cultured in PneumaCult™-Ex Plus Medium (Catalog #05040) and analyzed at passage three. Ciliated cell markers, FOXJ1 and TUBB4B were upregulated in terminally differentiated apical-out airway organoids, indicating robust differentiation across all three culture conditions. Slight upregulation of MUC5AC, a goblet cell marker, was detected in organoids generated with PneumaCult™ AOAOSM and was further increased with the addition of IL-13 (Catalog #78029). These results demonstrate that PneumaCult™ AOAOSM provides a flexible and reliable platform for modeling airway epithelial biology under both healthy and inflammatory conditions. Data points represent the mean from three independent wells of a 24-well plate (n = 3 donors).
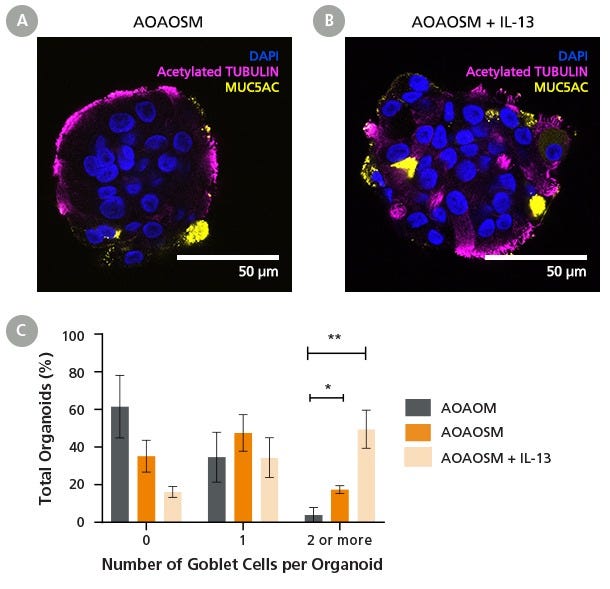
Figure 5. PneumaCult™ Apical-Out Airway Organoid Secretory Medium Supports Goblet Cell Differentiation
(A) Day 15 apical-out airway organoids cultured in PneumaCult™ Apical-Out Airway Organoid Secretory Medium (AOAOSM) contain outward-facing ciliated cells, as confirmed by the presence of acetylated tubulin (magenta), as well as goblet cells expressing MUC5AC (yellow).
(B) Addition of IL-13 in the medium appears to further induce goblet cell differentiation while allowing ciliogenesis, demonstrating the model’s responsiveness to cytokine-driven inflammatory cues. Images show representative organoids generated from human bronchial epithelial cells (HBECs) at passage 4.
(C) Analysis of MUC5AC-positive goblet cells per organoid reveals that goblet cell presence is minimal in cultures grown in PneumaCult™ Apical-Out Airway Organoid Medium (AOAOM; Catalog #100-0620), with most organoids lacking MUC5AC expression (61.49% ± 13.5%). In contrast, PneumaCult™ AOAOSM supports greater goblet cell differentiation, with 47.49% ± 7.95% of organoids containing one goblet cell, and 17.33% ± 1.69% containing two or more. The addition of IL-13 further promotes goblet cell formation, with 34.34% ± 8.63% of the organoids having at least one MUC5AC-positive cell, and 49.49% ± 8.26% of organoids containing two or more. At least 120 Day 28 organoids were imaged per condition for every donor (n = 3). These results demonstrate that PneumaCult™ AOAOSM provides a robust and flexible platform that allows researchers to investigate goblet cell dynamics in health and disease models.
Find supporting information and directions for use in the Product Information Sheet or explore additional protocols below.
This product is designed for use in the following research area(s) as part of the highlighted workflow stage(s). Explore these workflows to learn more about the other products we offer to support each research area.
Thank you for your interest in IntestiCult™ Organoid Growth Medium (Human). Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration
| Species | Human |
|---|---|
| Formulation Category | Serum-Free |
在气液界面培养的人气道上皮细胞的无血清和无bpe培养基
无血清和无bpe培养基用于原代人气道上皮细胞的扩增
在气液界面培养的人小气道上皮细胞的无血清和无bpe培养基

无血清和无bpe培养基用于气道类器官的高效建立和分化
无血清和无bpe培养基用于人原代支气管上皮细胞或人气道上皮细胞分化为成熟的顶出气道类器官
人肺泡类器官扩增和分化的细胞培养基
无血清和无BPE培养基,用于人大气道、小气道和鼻上皮细胞的扩增
扫描二维码或搜索微信号STEMCELLTech,即可关注我们的微信平台,第一时间接收丰富的技术资源和最新的活动信息。
如您有任何问题,欢迎发消息给STEMCELLTech微信公众平台,或与我们通过电话/邮件联系:400 885 9050 INFO.CN@STEMCELL.COM。