产品号 #200-0561_C
细胞培养板,用于简便且可重复生成类器官
细胞培养板,用于简便且可重复生成类器官
使用类器官培养板可简化类器官研究流程,解决传统基质包埋培养中常见的类器官培养挑战,例如差异性大、增长不一致等问题。该板旨在增强一致性,确保均匀的基质厚度,减少半月板效应,从而实现更轻松快速的接种、更清晰的成像,以及更可靠的实验结果。类器官将以更可预测的方式生长,帮助您更高效地工作,并增强对数据的信心。
优化的孔设计减少了边缘效应,并免去预热步骤,节省时间,并确保每个孔都可用,从而提高了每次实验的表现。与康宁®Matrigel®和其他矩阵以及自动化系统兼容,该板完全适合高通量工作流程,使其成为药物筛选或疾病建模等大规模研究的理想解决方案。在大小、形状、生长和成熟方面一致的类器官,有助于构建更具生理相关性的模型,更真实地模拟体内环境,适用于研究复杂疾病机制、测试治疗反应和推进大规模体外建模的理想选择。
类器官培养板适用于大多数传统包埋于基质穹顶中的类器官培养流程,包括使用Interticult™类器官生长培养基(人)和Interticult™类器官分化培养基(人)培养肠类器官,或使用Hepatcult™类器官生长培养基(人)和Hepatcult™类器官分化培养基(人)培养肝类器官。.培养的肝类器官。
亚型
培养皿与培养板
细胞类型
肝细胞,肠道细胞
应用
细胞培养,鉴定,分化,类器官培养,球状体培养,细胞毒性检测
研究领域
疾病建模,药物发现和毒理检测,上皮细胞研究,神经科学,类器官
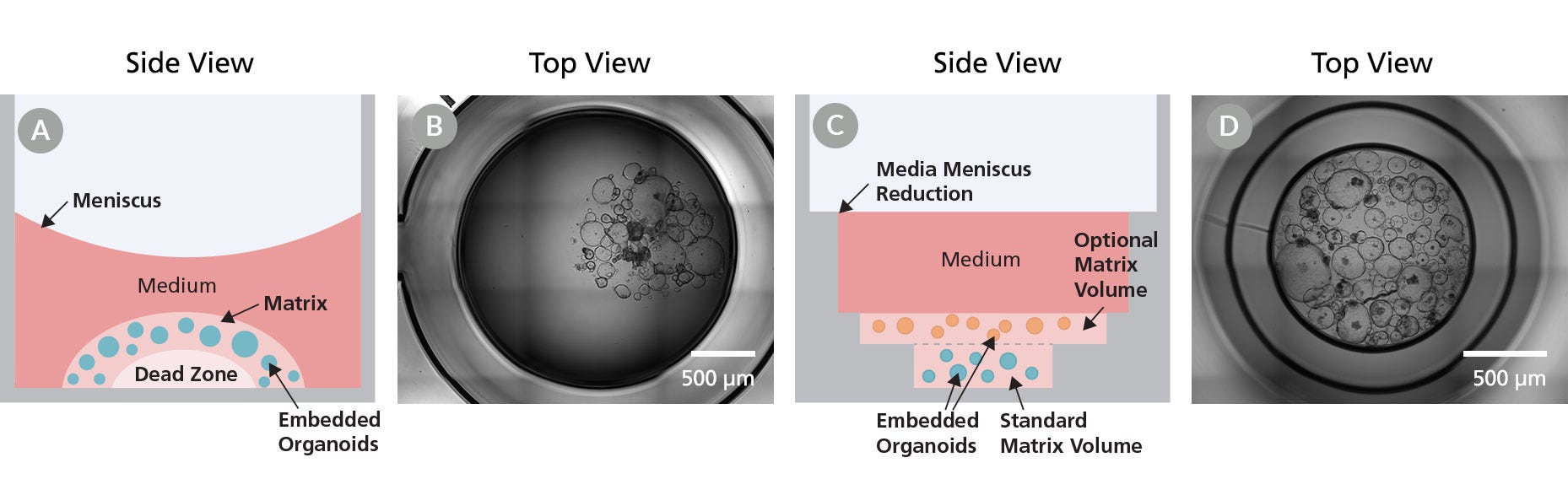
Figure 1. Organoid Culture Plates Enable Superior Imaging and Ease of Use Over Dome Culture in Standard Culture Plates
(A,B) Challenges associated with organoids cultured in matrix domes using standard culture plates include inconsistencies by manually placing domes in the center of the well, culture “dead zones” with poor or no growth, variability in dome shape, height, and position, the inability to form domes from diluted matrix, and imaging impairment due to the meniscus. (C,D) The stepped features of the Organoid Culture Plates support a standardized matrix depth, reduced meniscus, and increased culture robustness resulting in more uniform growth, uniform organoid position, and fewer failed wells. Additionally, an optional second matrix volume enables layered cultures or co-cultures. Taken together, Organoid Culture Plates enable increased user efficacy, as well as clearer imaging for more accurate observations to benefit detailed studies and both quantitative and qualitative data collection.
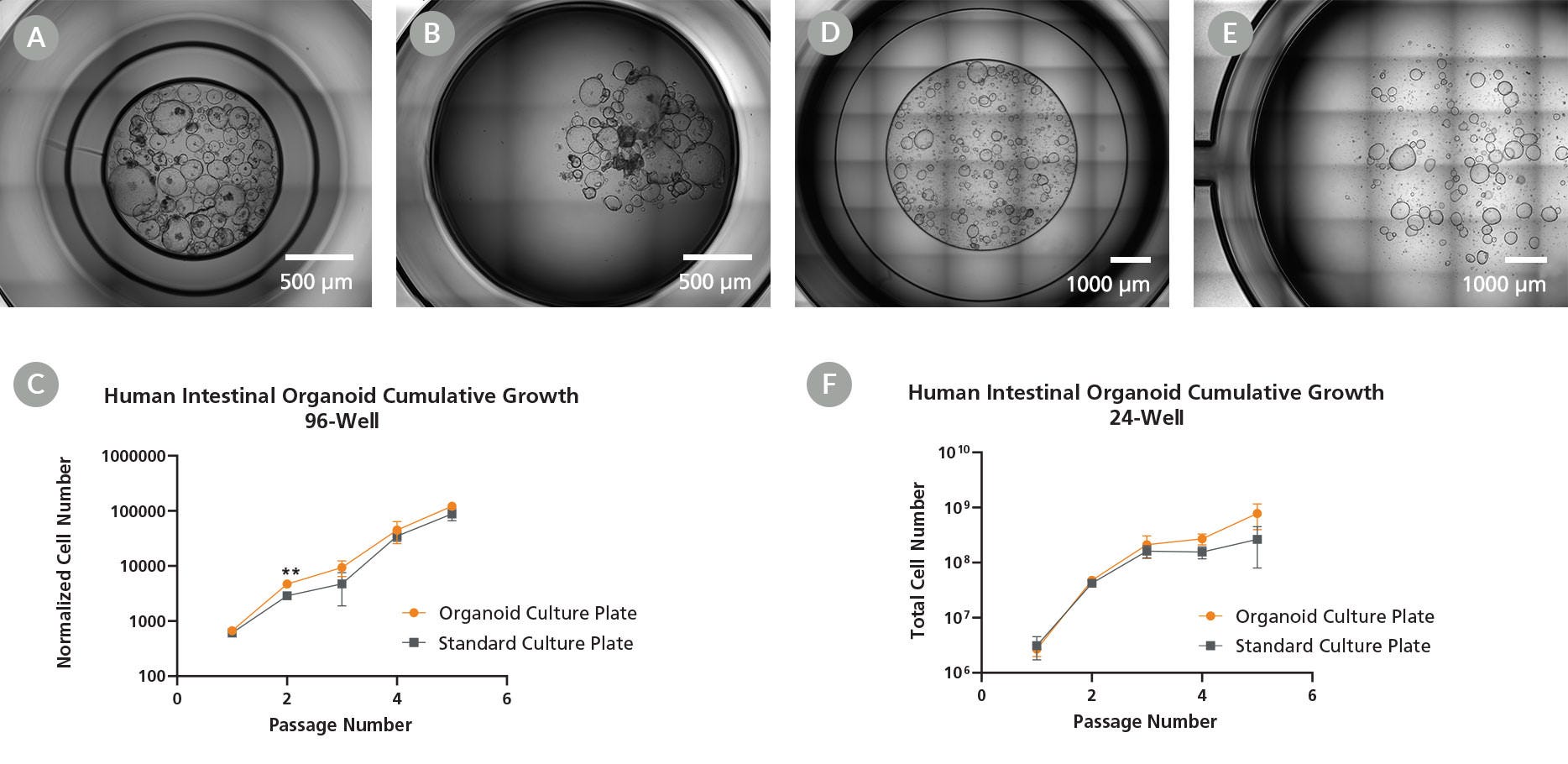
Figure 2. Organoid Culture Plates Support Equivalent Cell Growth in Addition to Enhanced Imaging
Organoid morphology and growth in the Organoid Culture Plates were assessed against dome culture in standard culture plates in both (A,B,C) 96- and (D,E,F) 24-well plate formats. Brightfield images show expected cystic morphology in human intestinal organoids cultured in the (A) 96-well Organoid Culture Plate and (B) 96-well standard culture plate after 7 days. (C) Human intestinal organoid growth was assessed in the 96-well Organoid Culture Plates or standard culture plates over 5 passages. The cell number was normalized to the number of fragments at Day 0 to account for different seeding volumes between the 96-well Organoid Culture Plates and the 96-well standard culture plates. The Organoid Culture Plates had a significantly higher normalized cell number at passage 2 but were otherwise equivalent in their capacity to support cellular growth when compared to standard culture plates (n = 3). Brightfield images show expected cystic morphology in human intestinal organoids culture in the (D) 24-well Organoid Culture Plate and (E) 24-well standard standard culture plates after 7 days. (F) Human intestinal organoid growth was assessed in the 24-well Organoid Culture Plates or in standard culture plates over 5 passages, demonstrating equivalent capacity to support cellular growth between culture plates (n = 3), ** p < 0.01 Significance determined using a mixed-effects analysis with Sidak’s test for multiple comparisons.
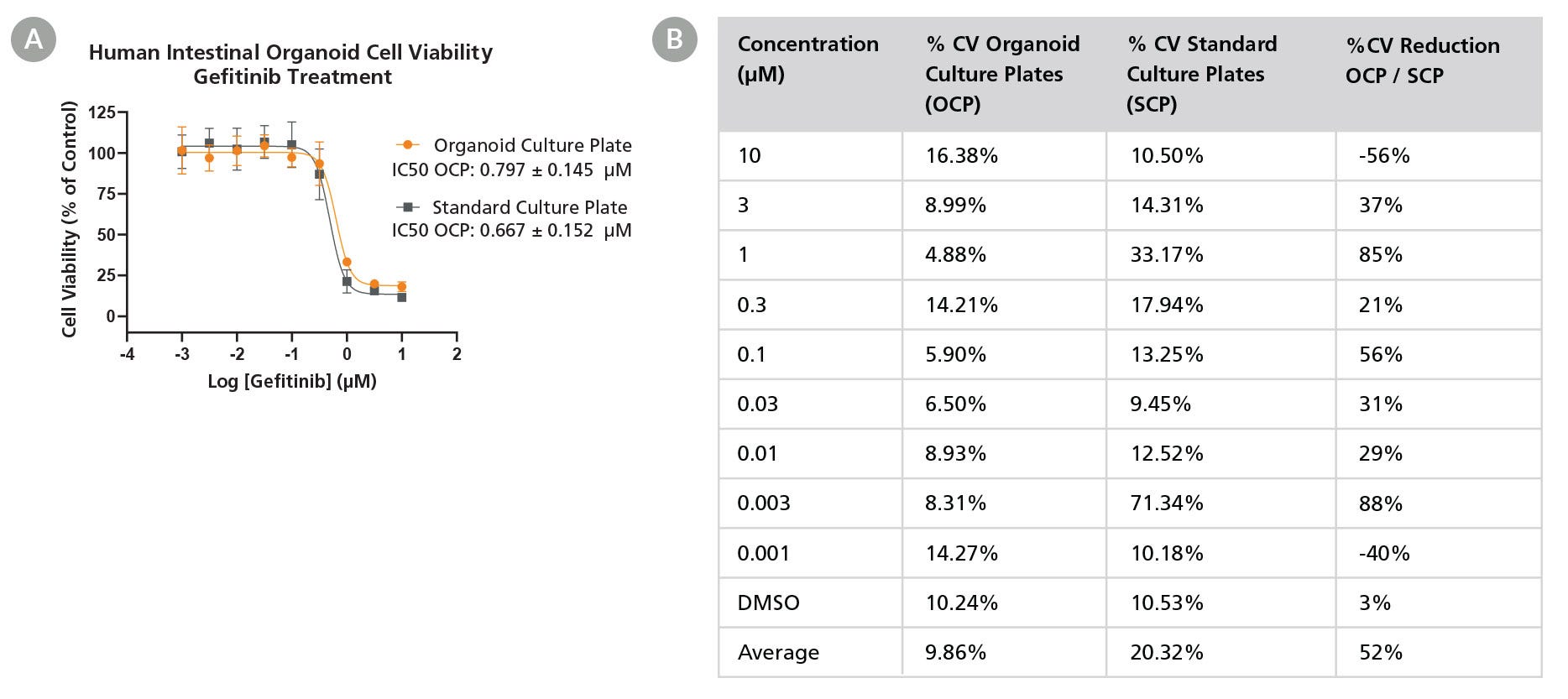
Figure 3. Organoid Culture Plates Enhance Consistency in Toxicity Screening by Providing a Standardized Platform
Human-derived intestinal organoids were cultured in 96-well Organoid Culture Plates or 96-well standard culture plates for 2 days, followed by Gefitinib treatment for an additional 4 days. Relative cell viability was assessed on Day 6 via CellTiterGloⓇ 3D analysis. (A) Screening cultures in Organoid Culture Plates produced similar IC50 values to organoid domes in standard culture plates after Gefitinib treatment (0.797 ± 0.145 µM and 0.667 ± 0.152 µM respectively). (B) Organoids cultured in the Organoid Culture Plates had a lower coefficient of variation in cell viability between technical replicates following the Gefitinib treatment compared to the dome cultures from the standard culture plates, resulting in higher confidence and more robust data. %CV = Percent coefficient of variation. OCP = Organoid Culture Plate. SCP = Standard Culture Plate. %CV reduction was calculated using 1 - %CV Organoid Culture Plate / %CV Standard Culture Plate
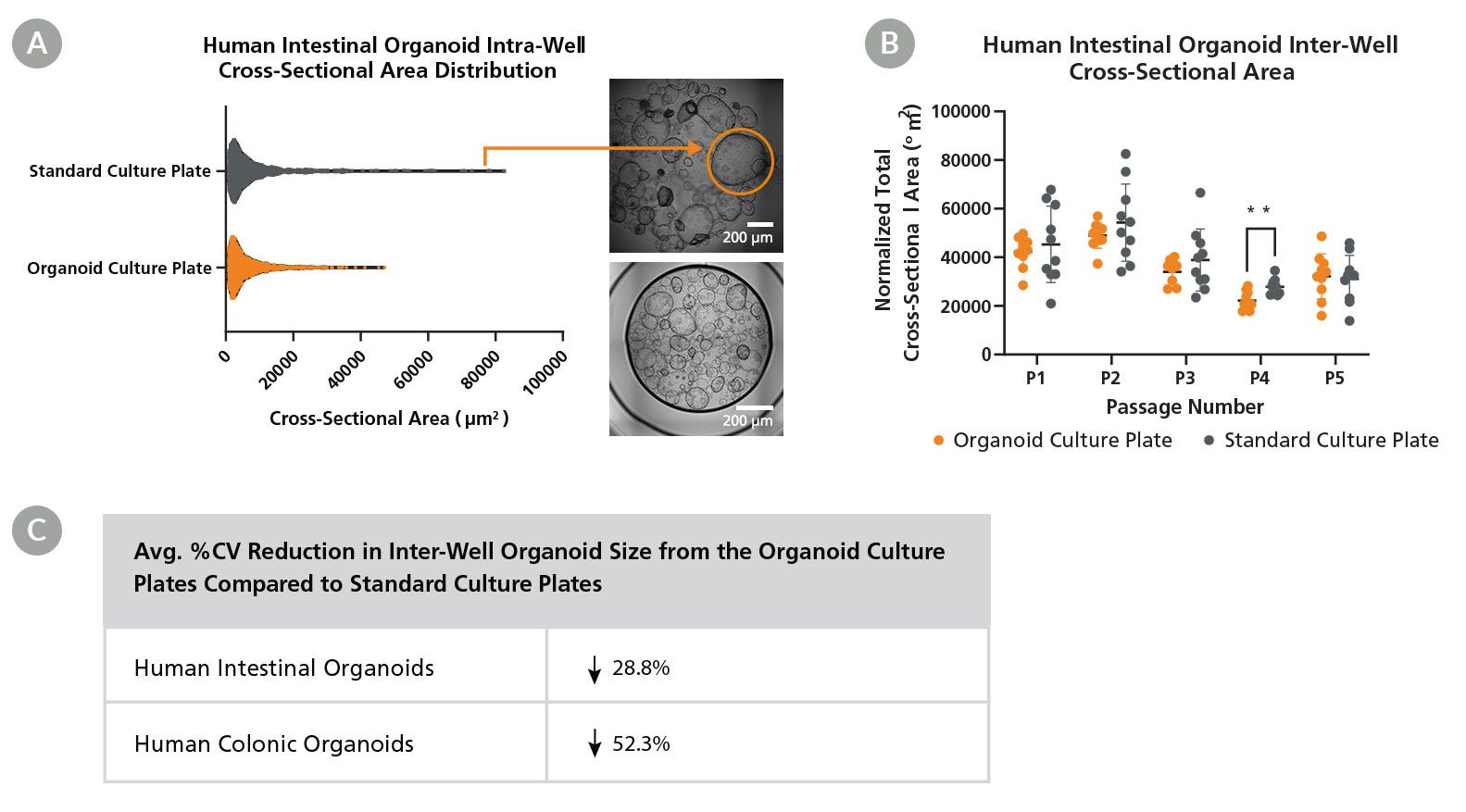
Figure 4. Organoid Culture Plates Reduce Intra-Well and Inter-Well Heterogeneity for Increased Consistency in Organoid Size
(A) Within a single well, human intestinal organoids cultured in the 96-well Organoid Culture Plates have a reduced range of organoid cross-sectional area when compared to human intestinal organoids cultured in standard culture plates. (B) For inter-well comparisons, organoids cultured in the Organoid Culture Plates exhibited lower variability in cross-sectional area compared to the organoid cultured in standard culture plates, as indicated by the reduced spread of the data. (C) The coefficient of variation (%CV) was used to measure consistency and reproducibility in the inter-well organoid cross-sectional area between culture plate formats, demonstrating reduced inter-well organoid size for organoids cultured in the Organoid Culture Plates. %CV reduction was calculated using 1 - %CV Organoid Culture Plate/%CV Standard Culture Plate
Find supporting information and directions for use in the Product Information Sheet or explore additional protocols below.
Thank you for your interest in IntestiCult™ Organoid Growth Medium (Human). Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration
用于建立和维持人肠道类器官的培养基
用于将人肠道类器官在3D,或在单层/气液界面培养的形式下进一步分化的培养基
用于人肝脏类器官生成、生长和分化的培养基试剂盒
扫描二维码或搜索微信号STEMCELLTech,即可关注我们的微信平台,第一时间接收丰富的技术资源和最新的活动信息。
如您有任何问题,欢迎发消息给STEMCELLTech微信公众平台,或与我们通过电话/邮件联系:400 885 9050 INFO.CN@STEMCELL.COM。