产品号 #09701_C
用于测定药物红系造血毒性的无血清培养基及补充剂
该产品为筛选大量新药化合物对人造血干祖细胞(HSPC)增殖和往红系定向分化的影响而设计。
与使用集落形成单位(CFU)实验相比,基于液体培养的HemaTox™实验更加灵活:您可以在整个培养过程的不同时间点添加测试化合物,测定其对更原始或更成熟的祖细胞的影响。HemaTox™红系试剂盒促进CD34+ HSPC增殖以及分化为表达红系标志物(如CD71和CD235a)的细胞。
用户可以自选读出方法来量化细胞对每个待测化合物的反应以及估计化合物的IC50和IC90。每套试剂盒可在5块96孔板中测试多达160个条件(三复孔)。该试剂盒可单独使用或与HemaTox™髓系试剂盒(产品号 #09704)或HemaTox™巨核细胞试剂盒(产品号 #09707)联合使用,以平行评估谱系特异性药物造血毒性。
亚型
专用培养基
细胞类型
红系细胞,造血干/祖细胞
种属
人
应用
细胞培养,细胞毒性检测
品牌
HemaTox
研究领域
药物发现和毒理检测,干细胞生物学
制剂类别
无血清
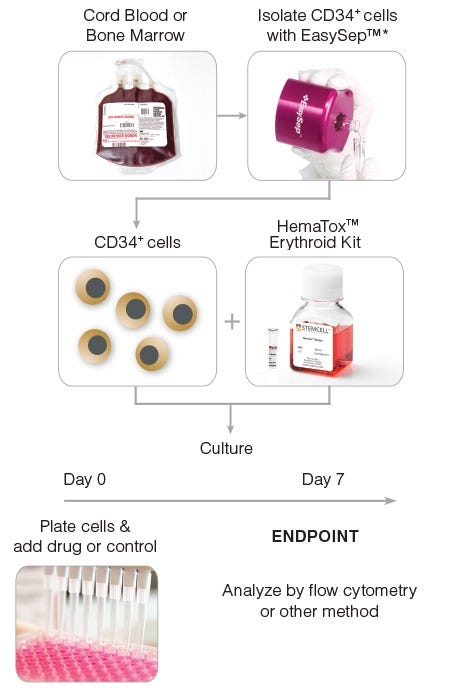
Figure 1. General HemaTox™ Erythroid Kit Procedure
*The cell isolation step may be omitted if pre-enriched CD34+ cells are used.
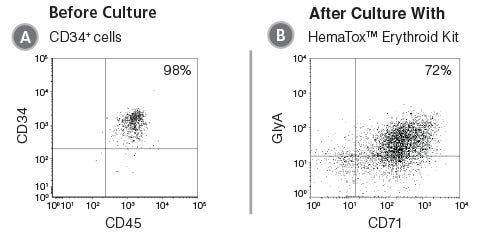
Figure 2. Flow Cytometry Plot Showing Erythroid Cells Produced After Culture of CD34+ HSPCs with the HemaTox™ Erythroid Kit
(A) Human CB CD34+ cells were cultured with the HemaTox™ Erythroid Kit using the protocol as written in the Product Information Sheet (PIS). (B) After the appropriate culture period, cells were harvested and stained for cell surface proteins expressed on erythroid (CD71 and GlyA) cells.
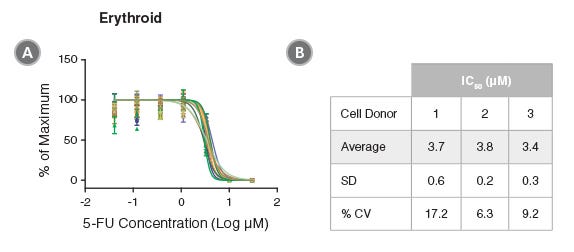
Figure 3. Reproducibility of HemaTox™ Erythroid Kit Results Between Experiments and Using Different CD34+ Cell Preparations
(A) Dose-response curves were generated from titrations of 5-FU added to human CB CD34+ cells isolated from 3 donors and cultured with the HemaTox™ Erythroid Kit. Three to five separate experiments were performed with cells from each donor. In each assay similar IC50 values were obtained with cells from different donors and in different experiments with cells from the same donor. Shown are values normalized to the percentages (%) of maximum cell growth without drug. Despite differences in absolute cell counts, curves are reproducible when normalized within each experiment. (B) Table showing IC50 values generated for 5-FU in culture with the erythroid kit including standard deviation (SD) and the coefficient of variation (% CV) across experiments.
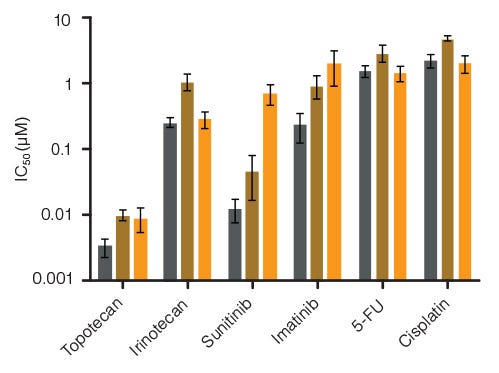
Figure 4. Lineage-Specific Differences in Hematotoxicity Identified with HemaTox™ Erythroid, Myeloid and Megakaryocyte Kits
Results show average IC50 values for each drug tested on human BM CD34+ cells using the HemaTox™ Erythroid (grey), Myeloid (gold) and Megakaryocyte (orange) Kits. Most drugs show similar toxicity for each lineage but some, such as Sunitinib, are ~100-fold more toxic for erythroid than for megakaryocyte progenitor differentiation with intermediate toxicity for myeloid progenitor differentiation. Vertical lines indicate standard error of the mean (SEM) (n = 4 - 8).
Find supporting information and directions for use in the Product Information Sheet or explore additional protocols below.
Thank you for your interest in IntestiCult™ Organoid Growth Medium (Human). Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration
| Species | Human |
|---|---|
| Formulation Category | Serum-Free |
用于测定药物髓系造血毒性的无血清培养基及补充剂
用于测定药物巨核细胞造血毒性的无血清培养基及补充剂

小鼠单克隆IgG1抗体,抗人CD71(转铁蛋白受体)
扫描二维码或搜索微信号STEMCELLTech,即可关注我们的微信平台,第一时间接收丰富的技术资源和最新的活动信息。
如您有任何问题,欢迎发消息给STEMCELLTech微信公众平台,或与我们通过电话/邮件联系:400 885 9050 INFO.CN@STEMCELL.COM。