产品仅供研究使用,除另有说明外,不得用于人类或动物的诊断或治疗用途。有关 STEMCELL 质量的更多信息,请访问 www.stemcell.com/compliance。
概述
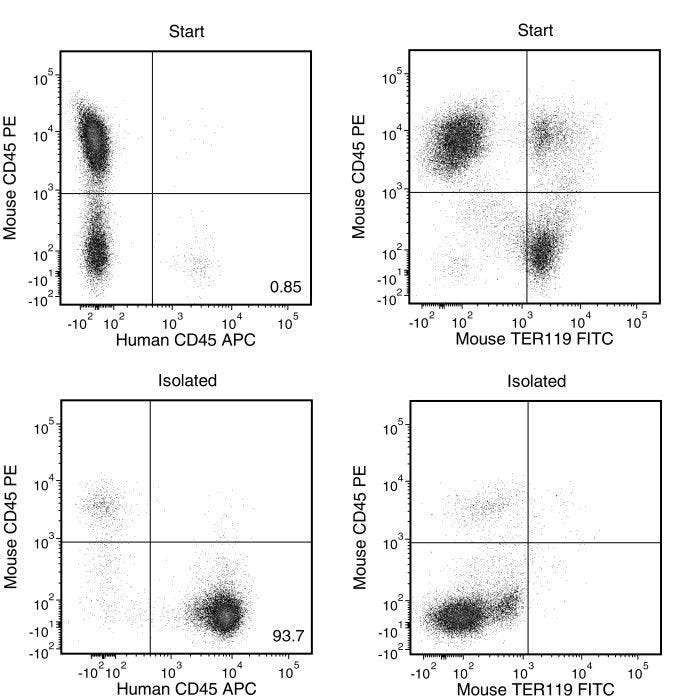
EasySep™小鼠/人嵌合体分选试剂盒旨在通过负选方法,从人源化移植受体小鼠的骨髓、脾脏或外周血中高度富集人类细胞。利用针对小鼠造血细胞的生物素化抗体和链霉亲和素包被的磁珠,靶向去除不需要的细胞。通过被标记的细胞无需使用分离柱,使用EasySep™磁极即可,目的细胞可直接倒入新分离管中收集。该产品可替代EasySep™小鼠/人嵌合体富集试剂盒 (产品号 #13068) 以实现更快更简单的细胞分选。
MAGNET COMPATIBILITY
• EasySep™ Magnet (Catalog #18000)
• “The Big Easy” EasySep™ Magnet (Catalog #18001)
• EasyEights™ EasySep™ Magnet (Catalog #18103)
• EasyPlate™ EasySep™ Magnet (Catalog #18102)
SUBTYPE
Cell Isolation Kits
SPECIES
Mouse
SAMPLE SOURCE
Bone Marrow, Spleen, Whole Blood
SELECTION METHOD
Negative
APPLICATION
Cell Isolation
BRAND
EasySep
AREA OF INTEREST
Cancer, Immunology
实验数据
产品说明书及文档
Find supporting information and directions for use in the Product Information Sheet or explore additional protocols below.
相关材料与文献
Educational Materials (10)
Frequently Asked Questions
Publications (2)
Despite mutation acquisition in hematopoietic stem cells, JMML-propagating cells are not always restricted to this compartment. Leukemia 2020 jun
Abstract
Juvenile myelomonocytic leukemia (JMML) is a rare aggressive myelodysplastic/myeloproliferative neoplasm of early childhood, initiated by RAS-activating mutations. Genomic analyses have recently described JMML mutational landscape; however, the nature of JMML-propagating cells (JMML-PCs) and the clonal architecture of the disease remained until now elusive. Combining genomic (exome, RNA-seq), Colony forming assay and xenograft studies, we detect the presence of JMML-PCs that faithfully reproduce JMML features including the complex/nonlinear organization of dominant/minor clones, both at diagnosis and relapse. Further integrated analysis also reveals that although the mutations are acquired in hematopoietic stem cells, JMML-PCs are not always restricted to this compartment, highlighting the heterogeneity of the disease during the initiation steps. We show that the hematopoietic stem/progenitor cell phenotype is globally maintained in JMML despite overexpression of CD90/THY-1 in a subset of patients. This study shed new lights into the ontogeny of JMML, and the identity of JMML-PCs, and provides robust models to monitor the disease and test novel therapeutic approaches.
Genetically Engineered Cell-Derived Nanoparticles for Targeted Breast Cancer Immunotherapy. Molecular therapy : the journal of the American Society of Gene Therapy 2019 nov
Abstract
Exosomes are nanosized membranous vesicles secreted by a variety of cells. Due to their unique and pharmacologically important properties, cell-derived exosome nanoparticles have drawn significant interest for drug development. By genetically modifying exosomes with two distinct types of surface-displayed monoclonal antibodies, we have developed an exosome platform termed synthetic multivalent antibodies retargeted exosome (SMART-Exo) for controlling cellular immunity. Here, we apply this approach to human epidermal growth factor receptor 2 (HER2)-expressing breast cancer by engineering exosomes through genetic display of both anti-human CD3 and anti-human HER2 antibodies, resulting in SMART-Exos dually targeting T cell CD3 and breast cancer-associated HER2 receptors. By redirecting and activating cytotoxic T cells toward attacking HER2-expressing breast cancer cells, the designed SMART-Exos exhibited highly potent and specific anti-tumor activity both in vitro and in vivo. This work demonstrates preclinical feasibility of utilizing endogenous exosomes for targeted breast cancer immunotherapy and the SMART-Exos as a broadly applicable platform technology for the development of next-generation immuno-nanomedicines.