产品号 #100-1694_C
无动物源的添加物,用于人造血干细胞和祖细胞的扩增
StemSpan™HSC Plus添加物可增强造血干细胞和祖细胞(HSPC)研究工作流程——StemSpan™ HSC Plus添加物是一种经过精心配制的小分子混合物,与含细胞因子的培养基结合使用时,可提高造血干细胞(HSC)的产量。该配方经过优化,可支持多种工作流程,包括基因编辑、移植研究或扩增,从而提高细胞产量。在体外培养中,与含细胞因子的培养基结合使用时,StemSpan™ HSC Plus添加物可促进HSC的扩增,同时保留其功能潜力,帮助用户获得更高的产量和更稳定的结果。
将StemSpan™HSC Plus添加物可与以下任意StemSpan™培养基结合使用:
StemSpan™SFEM(产品号 #09600)
StemSpan™SFEM II(产品号 #09605)
StemSpan™-XF(产品号 #100-0073)
StemSpan™-AOF(产品号 #100-0130)
StemSpan™HSC Plus添加物须在添加了细胞因子的培养基中使用,并且已针对以下StemSpan™扩增添加物的使用进行了优化:
StemSpan™CD34扩增添加物(产品号 #02691)
StemSpan™CC100(产品号 #02690)
StemSpan™CC110(产品号 #02697)
细胞类型
造血干/祖细胞
品牌
StemSpan
研究领域
药物发现和毒理检测,细胞治疗开发
制剂类别
Animal Origin-Free,Phenol Red-Free
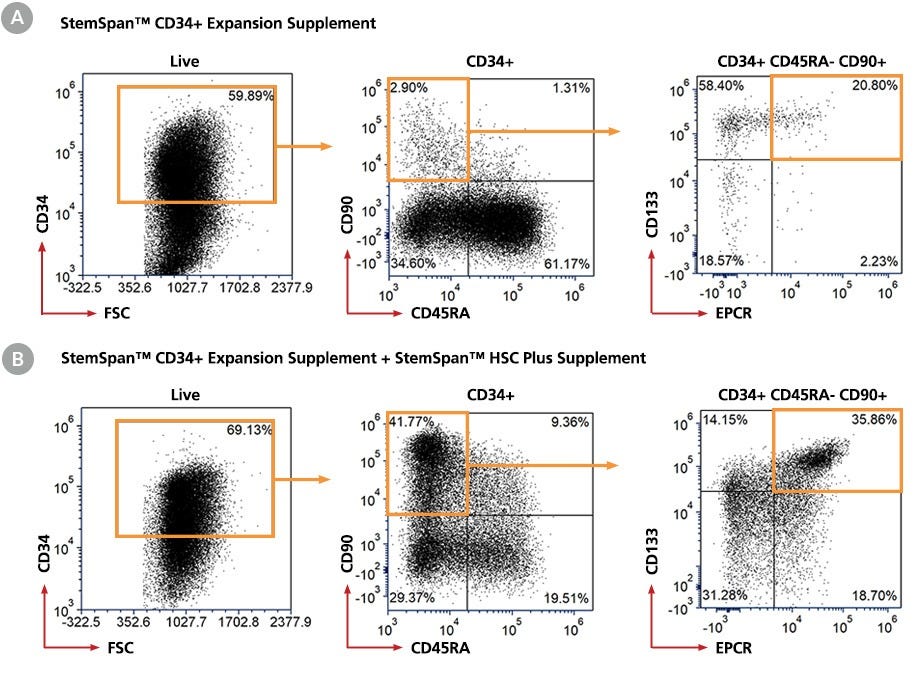
Figure 1. Gating Strategy for Immunophenotype Analysis of Cord Blood-Derived CD34+ Cells Expanded for 7 Days in StemSpan™ Media with and Without StemSpan™ HSC Plus Supplement
Purified CD34+ cells derived from cord blood (CB) were cultured for 7 days in StemSpan™ SFEM II medium supplemented with StemSpan™ CD34+ Expansion Supplement (A) alone or (B) together with StemSpan™ HSC Plus Supplement. After 7 days, the cultured cells were stained with fluorescently labeled antibodies against CD34, CD45RA, CD90, EPCR, and CD133, in addition to viability dye Zombie Yellow™, and analyzed by flow cytometry. Fluorescence minus one (FMO) controls were used to set gates for HSPCs subsets. Sequential gates (orange gates) were used to determine the percentages of viable CD34+ cells, CD34+ CD45RA- CD90+ cells, and CD34+CD45RA-CD90+CD133+EPCR+ cells.
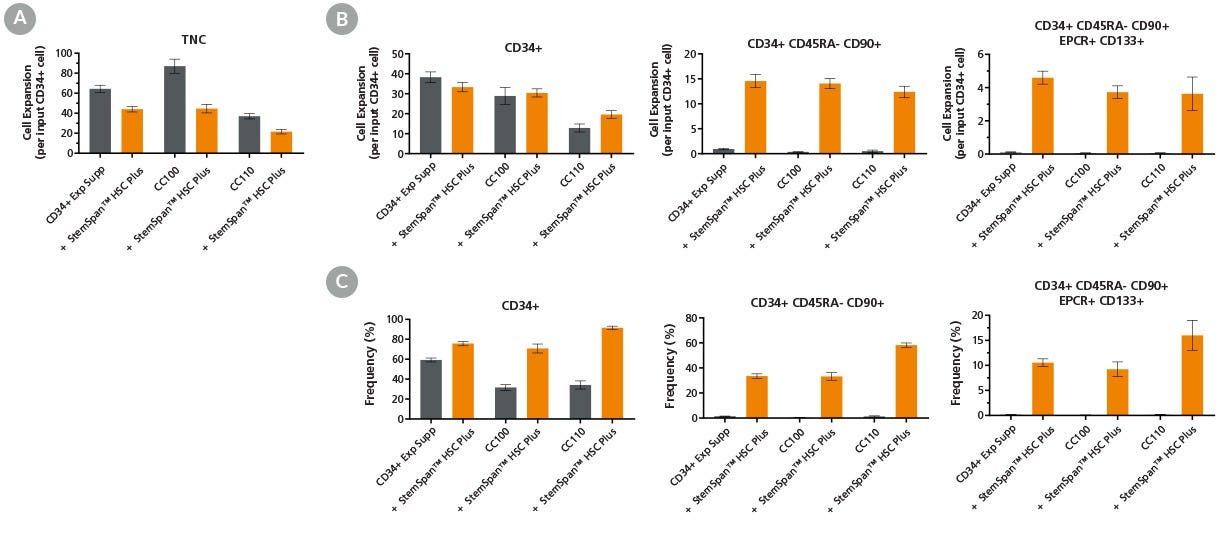
Figure 2. Expansion of Primitive HSPCs Is Enhanced by the Addition of StemSpan™ HSC Plus Supplement to Cytokine-Containing Medium
Purified CD34+ cells derived from cord blood (CB) were cultured at a concentration of 10,000 cells per mL in StemSpan™ SFEM II medium supplemented with one of the StemSpan™ cytokine-based supplements alone (CD34 Expansion Supplement, CC100, or CC110; gray bars), or together with StemSpan™ HSC Plus supplement (orange bars). After 7 days of culture, flow cytometry was used to analyze the (A) total nucleated cell (TNC) expansion, (B) expansion of CD34+ cell subsets, and (C) frequency of CD34+ HSPC subsets: CD34+, CD34+CD45RA-CD90+, and CD34+CD45RA-CD90+EPCR+CD133+. StemSpan™ HSC Plus Supplement promoted greater expansion of CD34+ CD45RA- CD90+ and CD34+CD45RA-CD90+CD133+EPCR+ HSPC subsets compared to cytokine-based supplements alone. Each subsequent cell subset was progressively more enriched for phenotypic stem/progenitor cells. Data shown are ± SEM (n = 24 for the CD34 Expansion Supplement dataset and n = 9 for both the CC100 and CC110 datasets).
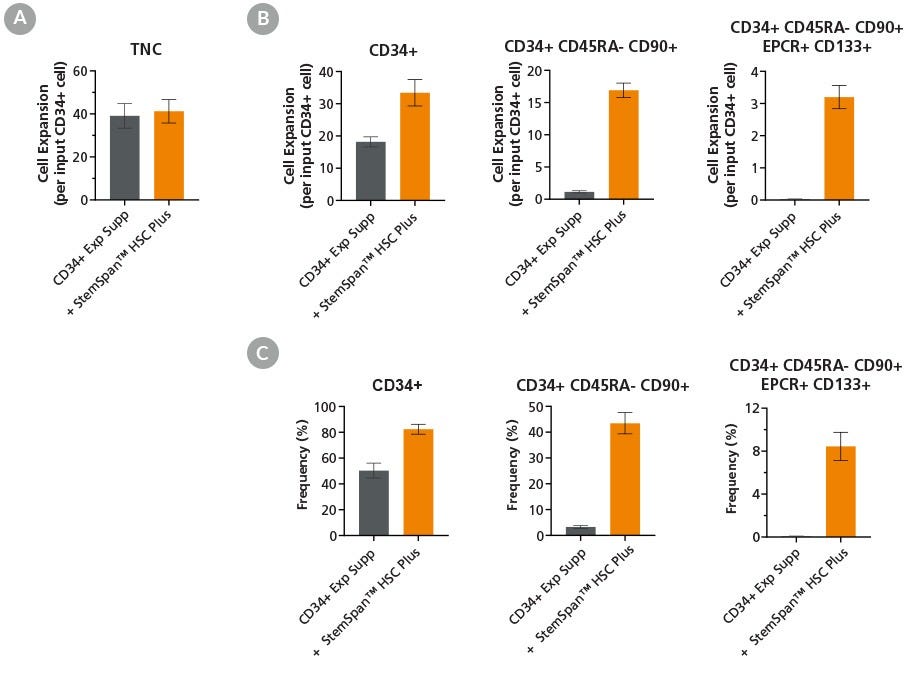
Figure 3. StemSpan™ HSC Plus Supplement Supports Expansion of Primitive HSPCs Derived from Mobilized Peripheral Blood
Purified CD34+ cells derived from G-CSF mobilized peripheral blood (mPB) were cultured at a concentration of 10,000 cells per mL in StemSpan™ SFEM II medium supplemented with StemSpan™ CD34 Expansion Supplement alone (gray bars) or together with StemSpan™ HSC Plus Supplement (orange bars). After 7 days of culture, flow cytometry was used to analyze the (A) total nucleated cell (TNC) expansion, (B) expansion of CD34+ cell subsets, and (C) frequency of CD34+ HSPC subsets: CD34+, CD34+CD45RA-CD90+ and CD34+CD45RA-CD90+EPCR+CD133+. StemSpan™ HSC Plus Supplement promoted higher expansion of CD34+ CD45RA- CD90+ and CD34+CD45RA-CD90+CD133+EPCR+ cells compared to StemSpan™ CD34 Expansion Supplement alone. Each subsequent cell subset was progressively more enriched for phenotypic stem/progenitor cells. Data shown are mean ± SEM (n = 6).

Figure 4. StemSpan™ HSC Plus Supplement Supports Expansion of Primitive HSPCs Derived from Bone Marrow
Purified CD34+ cells derived from bone marrow were cultured at a concentration of 10,000 cells per mL in StemSpan™ SFEM II medium supplemented with StemSpan™ CD34 Expansion Supplement alone (gray bars) or together with StemSpan™ HSC Plus Supplement (orange bars). After 7 days of culture, flow cytometry was used to analyze the (A) total nucleated cell (TNC) expansion, (B) expansion of CD34+ cell subsets, and (C) frequency of CD34+ HSPC subsets: CD34+, CD34+CD45RA-CD90+, and CD34+CD45RA-CD90+EPCR+CD133+. StemSpan™ HSC Plus Supplement promoted higher expansion of CD34+ CD45RA- CD90+ and CD34+CD45RA-CD90+CD133+EPCR+ cells compared to StemSpan™ CD34 Expansion Supplement alone. Each subsequent cell subset was progressively more enriched for phenotypic stem/progenitor cells. Data shown are mean ± SEM (n = 8).

Figure 5. StemSpan™ HSC Plus Supplement Supports Efficient Gene Knock-Out in Mobilized Peripheral Blood-Derived CD34+ Cells
Purified CD34+ cells derived from mobilized peripheral blood (mPB) were pre-cultured for 2 days in StemSpan™ SFEM II medium supplemented with StemSpan™ CD34 Expansion Supplement alone (dark gray bars) or together with StemSpan™ HSC Plus Supplement (orange bars). After two days, ~6.0 x 10^4 cells were electroporated using the Neon® Transfection System with RNP complexes targeting the B2M gene. Cells not exposed to the electroporation (untreated; light gray bars) were cultured with StemSpan™ CD34 Expansion Supplement and StemSpan™ HSC Plus Supplement.
(A) Four days post-electroporation, all conditions maintained >80% viability but (B) exhibited reduced cell recovery in RNP treated conditions. The frequency and yield of MHC-I- cells (i.e. B2M knock-out efficiency) in CD34+ cells and primitive CD34+CD45RA-CD90+EPCR+ HSPC subset were assessed via flow cytometry.
(C) The frequency and yield of B2M knock-out in CD34+ progenitor populations were similar between cultures with (orange bar) or without (dark gray) StemSpan™ HSC Plus Supplement.
(D) The yield of primitive CD34+CD45RA-CD90+EPCR+ gene-edited B2M knock-out cells was higher in cultures containing StemSpan™ HSC Plus Supplement. Data shown are mean ± SEM (n = 3).

Figure 6. Cord Blood CD34+ Cells Expanded with StemSpan™ HSC Plus Supplement Support Long-Term In Vivo Engraftment in Immunodeficient Mice
2,500 uncultured cord blood CD34+ cells or the progeny of 2,500 cord blood CD34+ cells cultured for 7 days under various conditions were transplanted into sub-lethally irradiated immunodeficient NSG mice. The cells were cultured in StemSpan™ SFEM II medium supplemented with StemSpan™ CD34 Expansion Supplement alone (dark gray bars) or together with StemSpan™HSC Plus Supplement (orange bars). Long-term multilineage engraftment was assessed in the bone marrow of transplanted NSG mice at 20 weeks post-transplantation. Data shown are mean ± SEM (n = 8 mice). Cells expanded with StemSpan™ HSC Plus Supplement demonstrated similar or higher levels of human engraftment in the bone marrow, as measured by frequency of (A) human CD45+ cells, (B) CD45+ CD34+ progenitor cells, (C) CD45+CD33+ myeloid cells, and (D) CD45+19+ B cells, relative to the other tested conditions. All transplanted cells showed lower or undetectable T cells engraftment (data not shown).
Find supporting information and directions for use in the Product Information Sheet or explore additional protocols below.
This product is designed for use in the following research area(s) as part of the highlighted workflow stage(s). Explore these workflows to learn more about the other products we offer to support each research area.
Thank you for your interest in IntestiCult™ Organoid Growth Medium (Human). Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration
| Formulation Category | Animal Origin-Free, Phenol Red-Free |
|---|
扫描二维码或搜索微信号STEMCELLTech,即可关注我们的微信平台,第一时间接收丰富的技术资源和最新的活动信息。
如您有任何问题,欢迎发消息给STEMCELLTech微信公众平台,或与我们通过电话/邮件联系:400 885 9050 INFO.CN@STEMCELL.COM。

