产品号 #100-0401_C
cGMP,无动物来源,稳定,无饲料的维持培养基,用于人类胚胎干细胞和iPS细胞
cGMP,无动物来源,稳定,无饲料的维持培养基,用于人类胚胎干细胞和iPS细胞
在您的人类多能干细胞衍生(hPSC)细胞治疗开发中,使用根据相关cgmp生产的TeSR™-AOF降低风险并获得更多高质量细胞。在二级生产阶段没有动物或人类来源的材料,与初级生产阶段只有动物来源的培养基相比,具有更直接的可追溯性和更高的病毒安全性。
使用TeSR™-AOF,无论您选择何种细胞系,都可以按照适合您的时间表持续培养病毒安全、高质量的psc。为了提高细胞质量属性,特别是在限制饲料期间,关键培养基成分已经稳定,包括成纤维细胞生长因子2 (FGF2);也称为碱性FGF [bFGF])。因此,TeSR™-AOF在保持细胞质量和等效性能的同时,允许每日和限制进料时间表。
TeSR™-AOF与多种培养基质兼容,包括康宁Matrigel hESC-Qualified Matrix(康宁目录#354277),Vitronectin XF™(目录#07180),以及CellAdhere™层粘连蛋白- 521(目录# 77003)。
在制造该介质或其成分时,不使用动物或人类来源的材料,至少达到二级制造水平。用于制备完整的TeSR™-AOF培养基的每批TeSR™-AOF 20X Supplement均在使用人多能干细胞的培养试验中进行性能测试。
TeSR™-AOF是在相关的cgmp下生产的,确保了可重复性结果的最高质量和一致性。了解更多关于质量和法规遵从性在干细胞的。
为TeSR™-AOF申请FDA主文件授权书(LOA),点击这里.
Subtype
Specialized Media
Cell Type
Pluripotent Stem Cells
Species
Human
Application
Cell Culture, Expansion, Maintenance
Brand
TeSR
Area of Interest
Disease Modeling, Drug Discovery and Toxicity Testing, Stem Cell Biology, Cell Therapy Development
Formulation Category
Animal Origin-Free
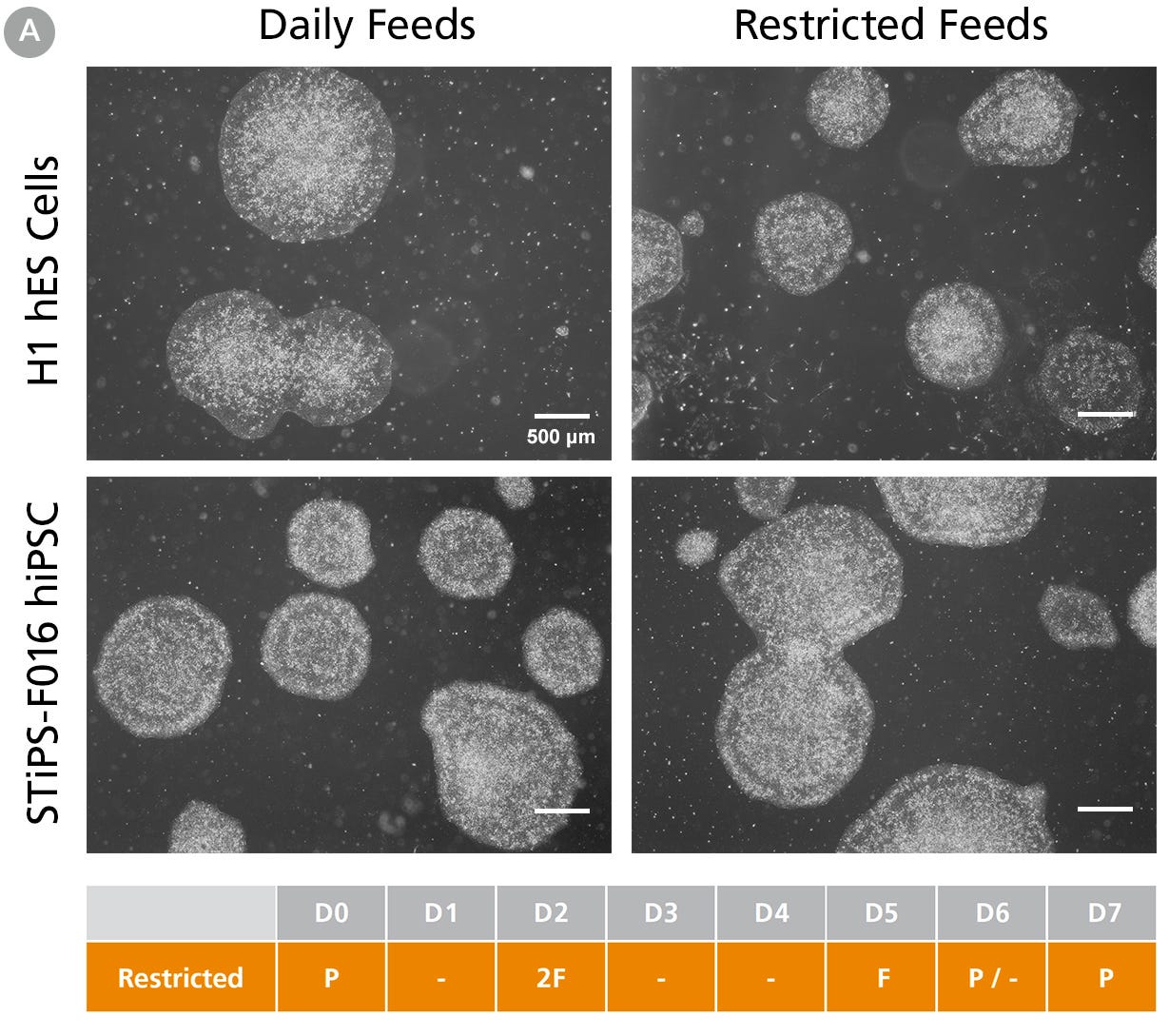
Figure 1A. hPSCs Maintained in TeSR™-AOF with Daily and Restricted Feed Schedules Exhibit Comparable Colony Morphology
hPSCs were maintained on Vitronectin XF™ for five passages. Phase-contrast images were taken on day 7 after seeding. For restricted feeds, hPSCs were fed with a double volume (4 mL) of medium on day 2 after passage, followed by two consecutive skipped days of feeds, with a final single-volume feed (2 mL) on day 5, prior to passaging on day 6 or 7.
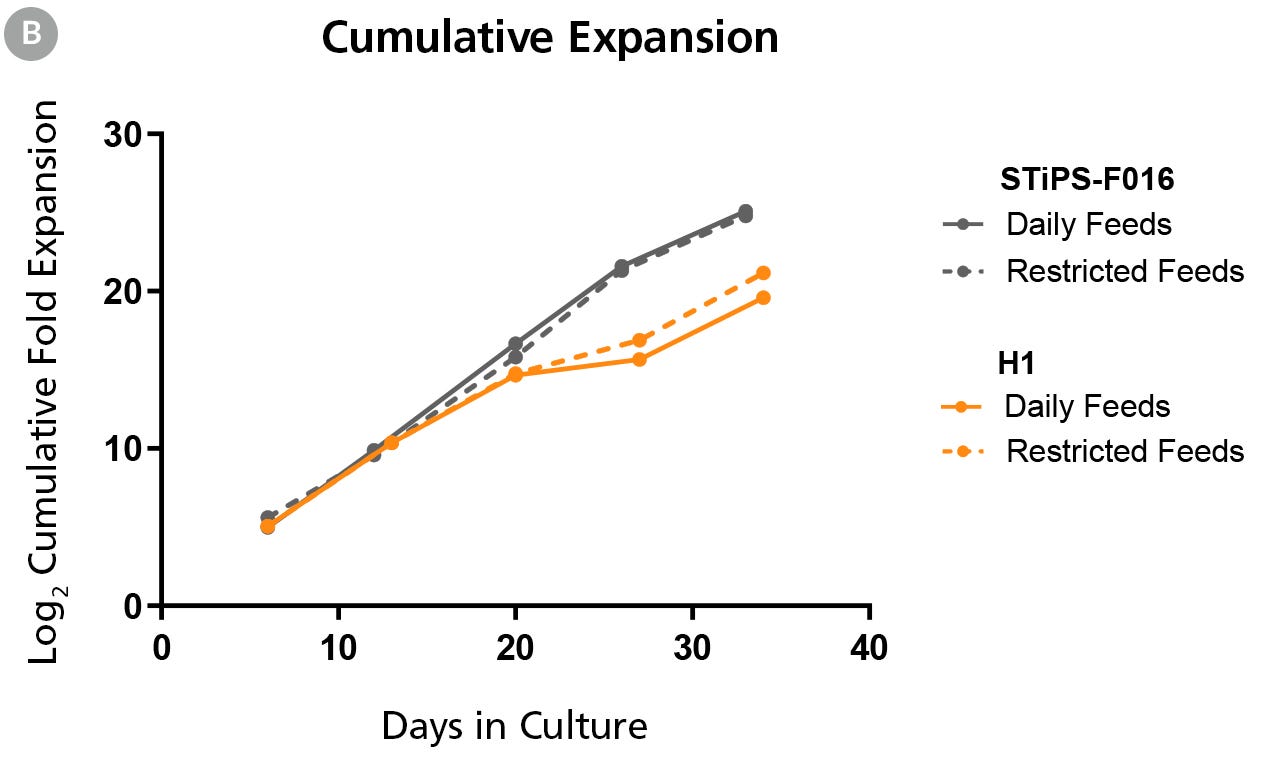
Figure 1B. hPSCs Maintained in TeSR™-AOF with Daily and Restricted Feed Schedules have Comparable Expansion Rates
hPSCs were maintained on Vitronectin XF™ for five passages. At the end of each passage cell counts were obtained using the Nucleocounter®️ NC-200 ChemoMetec automated cell counter to count DAPI-stained nuclei. The log2 transformed cumulative fold expansion was plotted against time in culture (days).
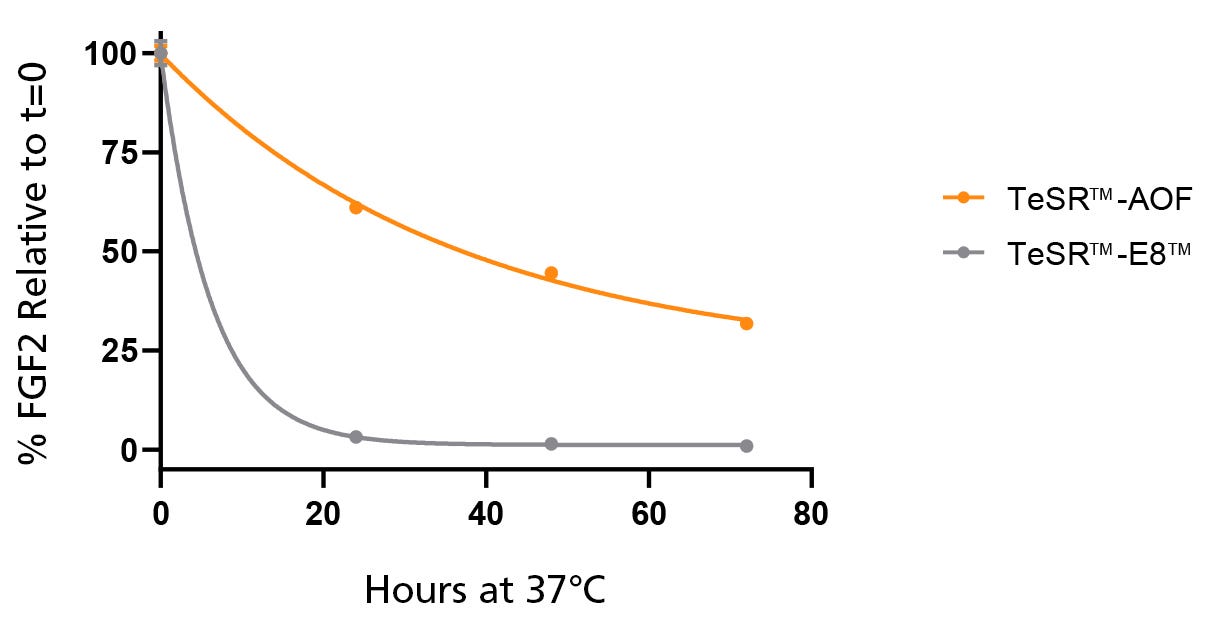
Figure 2. Native bFGF Levels are Stabilized at 37°C in TeSR™-AOF
TeSR™-AOF and TeSR™-E8™ were incubated at 37°C for 24, 48, and 72 hours. FGF2 levels were measured by Meso Scale Discovery (MSD) immunoassay; data was normalized to t = 0 levels for TeSR™-E8™ and TeSR™-AOF, respectively. FGF2 levels in TeSR™-AOF remain at 36.7 ± 5.61% of t = 0 levels at 72 hours when incubated at 37°C. Data representative of n = 3 biological replicates ± SD.
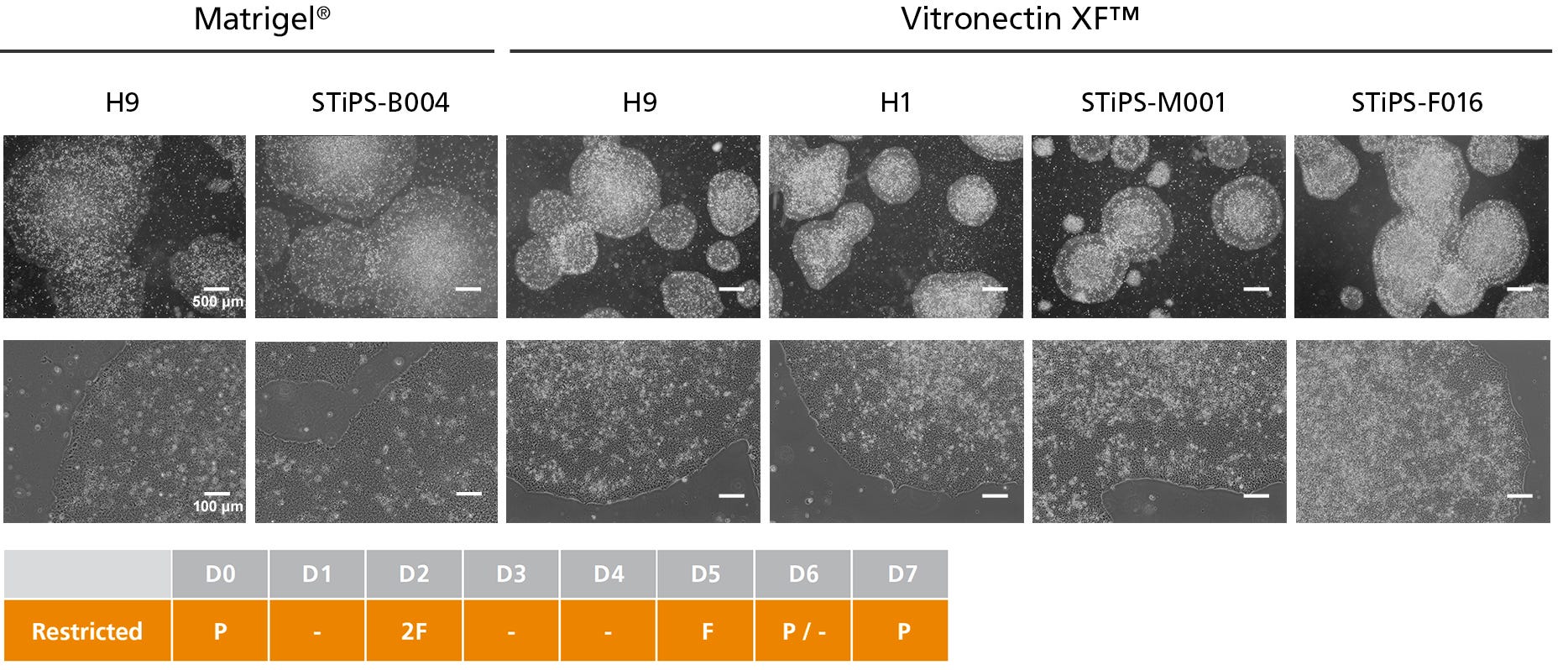
Figure 3. hPSCs Cultured in TeSR™-AOF with Restricted Feeding Maintain Demonstrate Classic hPSC Colony Morphology
hPSCs maintained in TeSR™-AOF were passaged as aggregates with ReLeSR™ passaging reagent every 6-7 days for greater than 10 passages. hPSCs maintained in TeSR™-AOF exhibit hPSC-like morphology, forming densely packed, round colonies with smooth edge morphology. Homogeneous cell morphology characteristic of hPSCs are observed, including large nucleoli and scant cytoplasm.
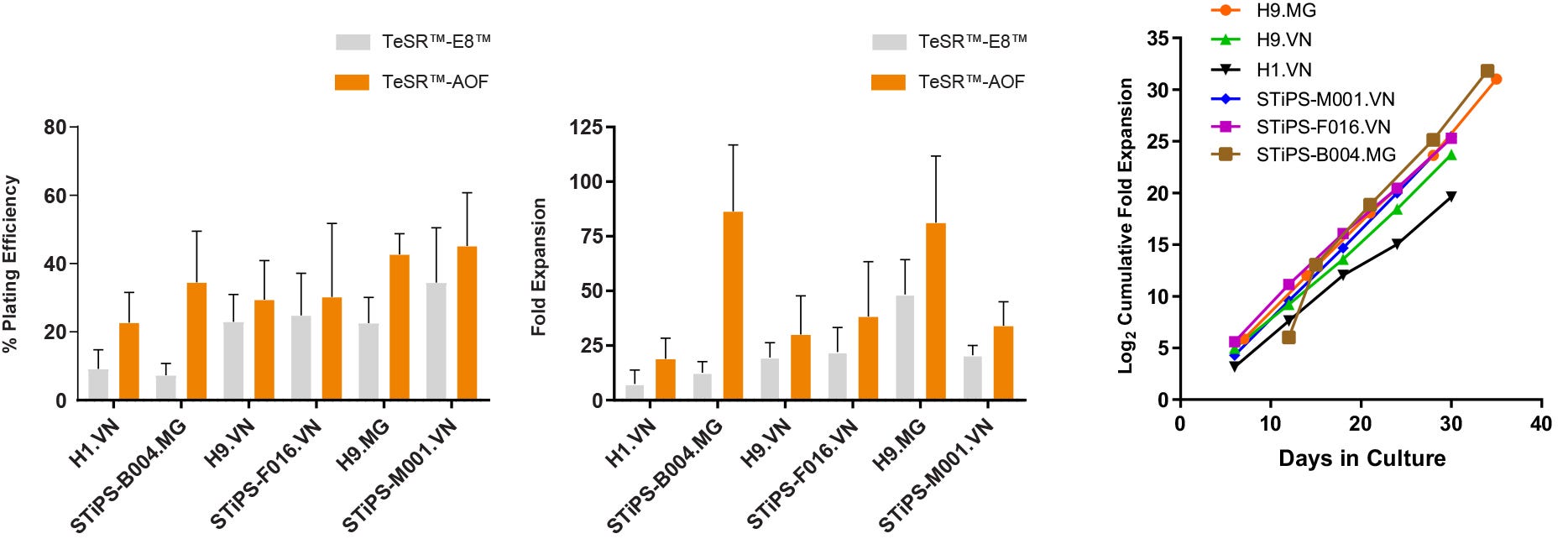
Figure 4. hPSCs Maintained in TeSR™-AOF Have Improved Attachment and Higher Overall Expansion Compared to Low-Protein Medium
(A) hPSCs cultured in TeSR™-AOF demonstrate a higher plating efficiency compared to hPSCs maintained in low-protein medium (TeSR™-E8™). Plating efficiency is calculated by seeding a known number of aggregates and comparing to the number of established colonies on day 7. (B) hPSCs maintained in TeSR™-AOF exhibit a higher average fold expansion per passage compared to TeSR™-E8™. (C) hPSCs cultured in TeSR™-AOF demonstrate consistent expansion and minimal cell line-to-cell-line variability between ES and iPS cell lines assessed. Cumulative fold expansion was measured from passage 1 to 5. Data represented as mean plating efficiency or fold expansion across 10 passages ± SD. MG = Matrigel®; VN = Vitronectin XF™.
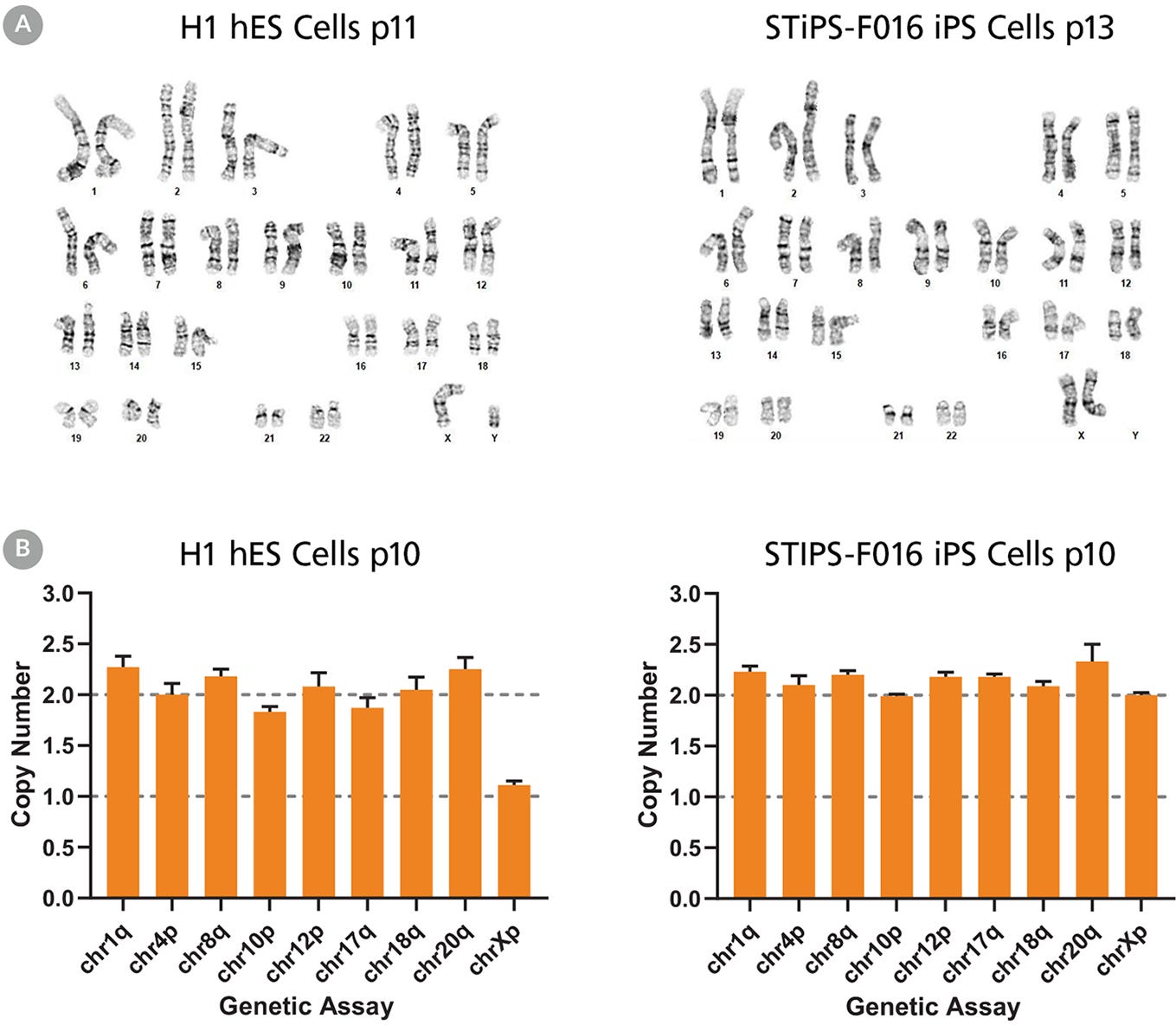
Figure 5. hPSCs Cultured in TeSR™-AOF with Restricted Feeding Maintain a Normal Karyotype
ES (H9 & H1) and iPS (STiPS-M001 & STiPS-F016) cell lines cultured in TeSR™-AOF were screened for chromosomal abnormalities using the hPSC Genetic Analysis Kit and by G-banding at ≥ 10 passages. Representative data are shown for (A) H1 ES and STiPS-F016 hiPS cell cultures at passage 10; no common chromosomal abnormalities were detected using the hPSC Genetic Analysis Kit, and (B) H1 ES cultures; these displayed a normal karyotype by G-banding at passage 11 and 13 respectively.
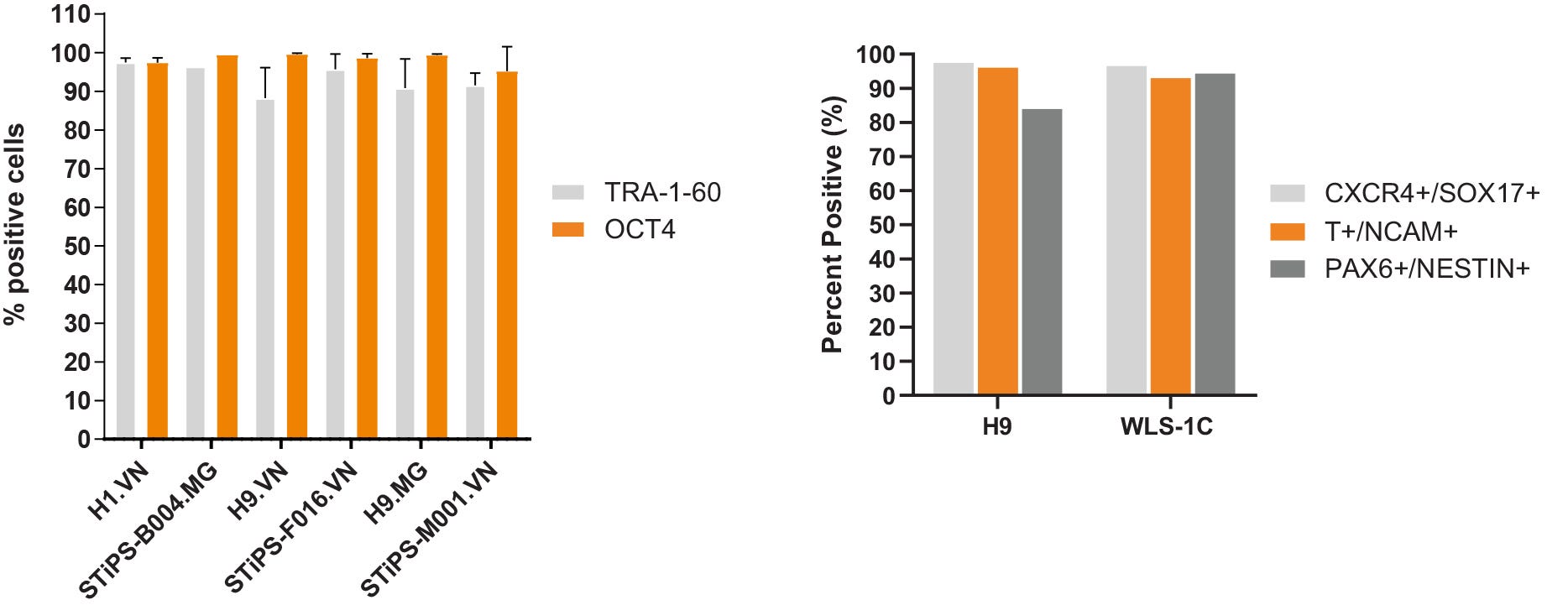
Figure 6. hPSCs Cultured in TeSR™-AOF Express Markers of the Undifferentiated State and Differentiate to the Three Germ Layers
(A) hPSCs maintained in TeSR™-AOF exhibit high levels of TRA-1-60 and OCT4 by flow cytometry at passage 5 and 10. Across n = 5 cell lines, the average TRA-1-60 expression was 92.8 ± 3.77 %, and percent OCT-4 positive cells were 98.1 ± 1.79%. Data shown represent an average of passage 5 and 10 flow results for each cell line. MG = Matrigel®; VN = Vitronectin XF™. (B) Efficient differentiation to the three germ layers was demonstrated in one hES and one hiPS cell line maintained for > 5 passages in TeSR™-AOF. Cultures were processed for flow cytometry and assessed for CXCR4+/SOX17+ cells on day 5 following differentiation using the STEMdiff™ Definitive Endoderm Kit. Cultures were processed for flow cytometry and assessed for Brachyury (T)+/OCT4- cells on day 5 following differentiation in STEMdiff™ Mesoderm Induction Medium. Cultures were processed for flow cytometry and assessed for PAX6+/Nestin+ cells on day 7 following monolayer differentiation using STEMdiff™ Neural Induction Medium.
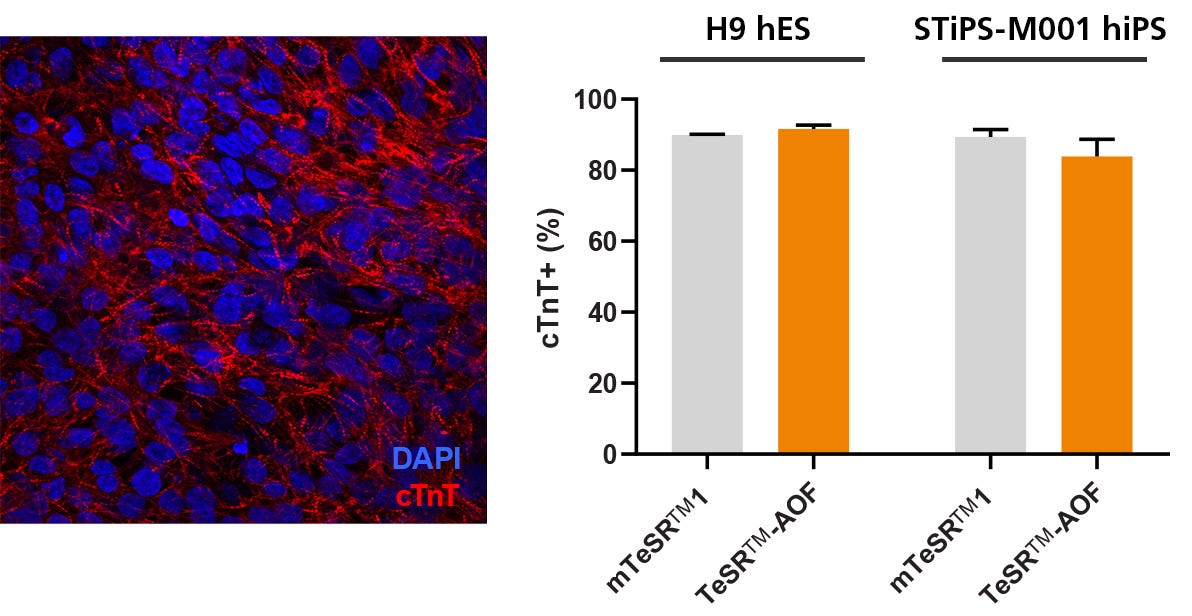
Figure 7. hPSCs Culture in TeSR™-AOF Differentiate to Cardiomyocytes with the STEMdiff™ Cardiomyocyte Differentiation Kit
Efficient differentiation to cardiomyocytes was demonstrated in 1 hES and 1 hiPS cell line maintained in TeSR™-AOF using the STEMdiff™ Cardiomyocyte Differentiation Kit. Expression of cardiac troponin T (cTnT) was assessed by immunocytochemistry (ICC) and by flow cytometry.
Find supporting information and directions for use in the Product Information Sheet or explore additional protocols below.
This product is designed for use in the following research area(s) as part of the highlighted workflow stage(s). Explore these workflows to learn more about the other products we offer to support each research area.
Thank you for your interest in IntestiCult™ Organoid Growth Medium (Human). Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration
| Species | Human |
|---|---|
| Formulation Category | Animal Origin-Free |
cGMP级、无酶的人多能干细胞选择与传代试剂
cGMP,无酶细胞解离试剂
成分明确的无异源基质,支持人多能干细胞在无血清、无饲养层条件下的生长和分化。

与 TeSR™ 维持培养基配合使用,用于维持人胚胎干细胞(ES)和诱导多能干细胞(iPS)的基质
扫描二维码或搜索微信号STEMCELLTech,即可关注我们的微信平台,第一时间接收丰富的技术资源和最新的活动信息。
如您有任何问题,欢迎发消息给STEMCELLTech微信公众平台,或与我们通过电话/邮件联系:400 885 9050 INFO.CN@STEMCELL.COM。