产品号 #100-0414_C
用于从生物液体中分离细胞外囊泡的粒径排除色谱柱
Dulbecco’s phosphate-buffered saline without calcium and magnesium

Non-sterile, clear, polypropylene snap-cap microcentrifuge tubes
Compatible antibodies for purity assessment of isolated cells
用细胞外囊泡大小隔离色谱(SEC)柱从多种生物基质中分离和纯化细胞外囊泡(ev),包括血浆、血清和细胞培养基。与其他EV分离方法相比,有效地将EV从循环蛋白中分离出来,而囊泡的改变(包括功能或大小)最小。SEC柱分离快速简便,随后ev适用于下游分析,如流式细胞术、western blot、核酸提取和/或功能分析。SEC柱有0.5 mL(10列/包),2 mL(5列/包)和20 mL(3列/包)大小,以支持不同的样品体积,可使用多达五次。
Subtype
Size Exclusion Chromatography Columns
Species
Human, Mouse, Non-Human Primate, Other, Rat
Application
Extracellular Vesicle Isolation
Area of Interest
Extracellular Vesicle Research
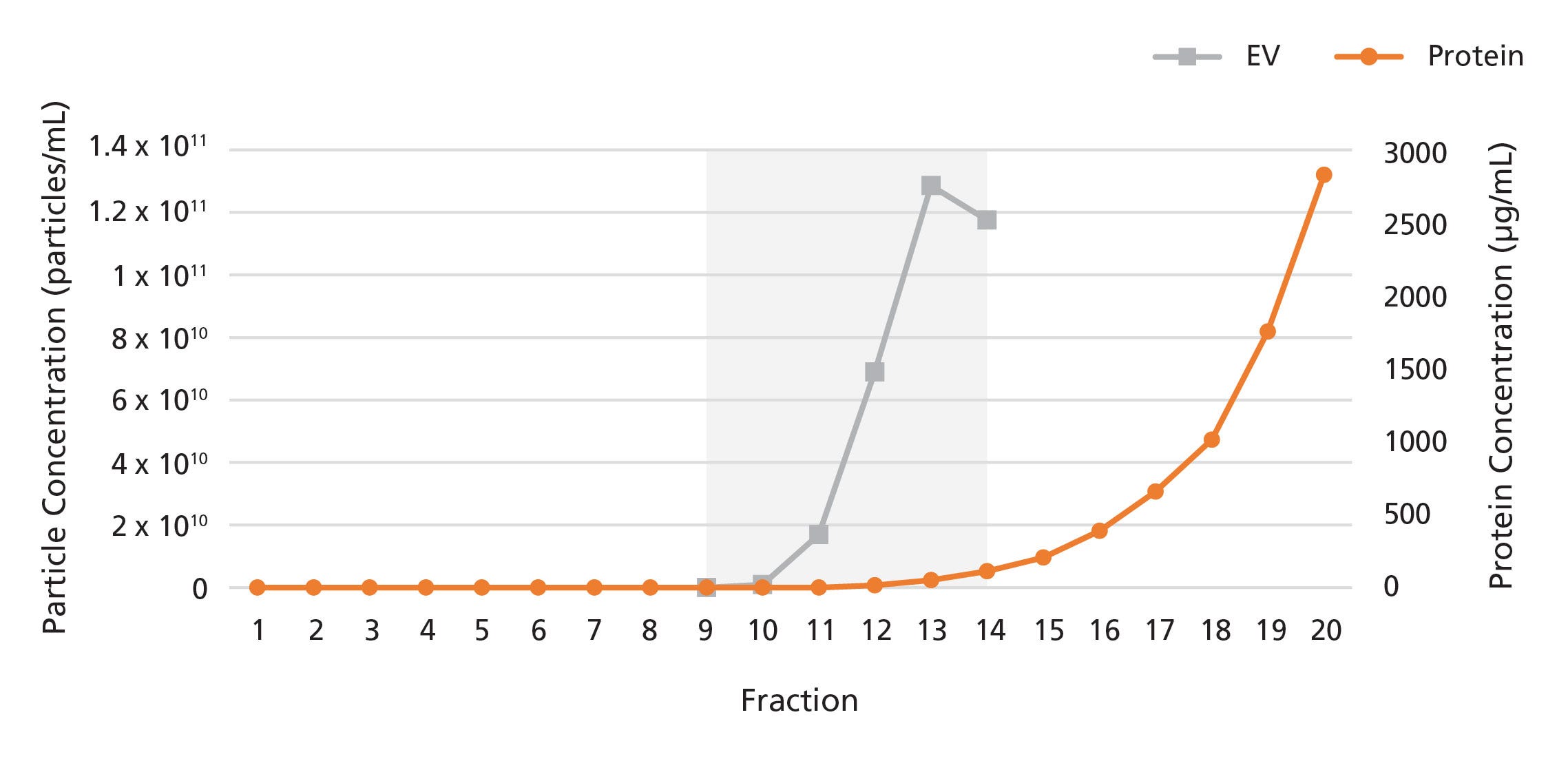
Figure 1. Isolation of Extracellular Vesicles from Plasma Using a 0.5 mL Extracellular Vesicle SEC Column
The 0.5 mL Extracellular Vesicle SEC Column was loaded with 0.5 mL of human plasma. 100 μL fractions were collected and analyzed for particle and protein content by nanoparticle tracking analysis (NTA) and bicinchoninic acid (BCA) assays, respectively. EVs were detected in fractions 9 - 14, while proteins were detected in fractions 15 onwards.
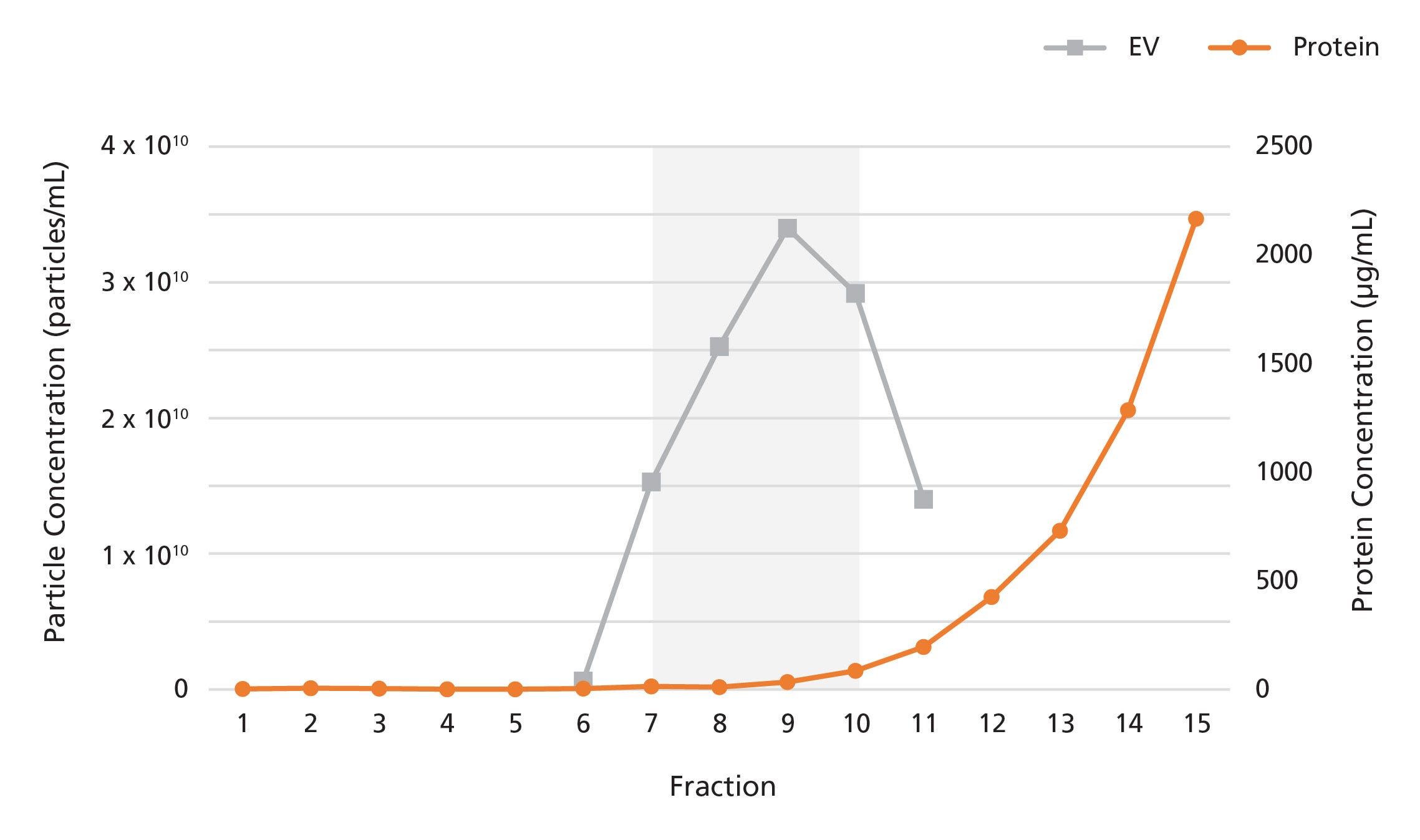
Figure 2. Isolation of Extracellular Vesicles from Plasma Using a 2 mL Extracellular Vesicle SEC Column
The 2 mL Extracellular Vesicle SEC Column was loaded with 2 mL of human plasma. 500 μL fractions were collected and analyzed for particle and protein content by nanoparticle tracking analysis and bicinchoninic acid assays, respectively. EVs were detected in fractions 7 - 10, while proteins were detected in fractions 11 onwards.
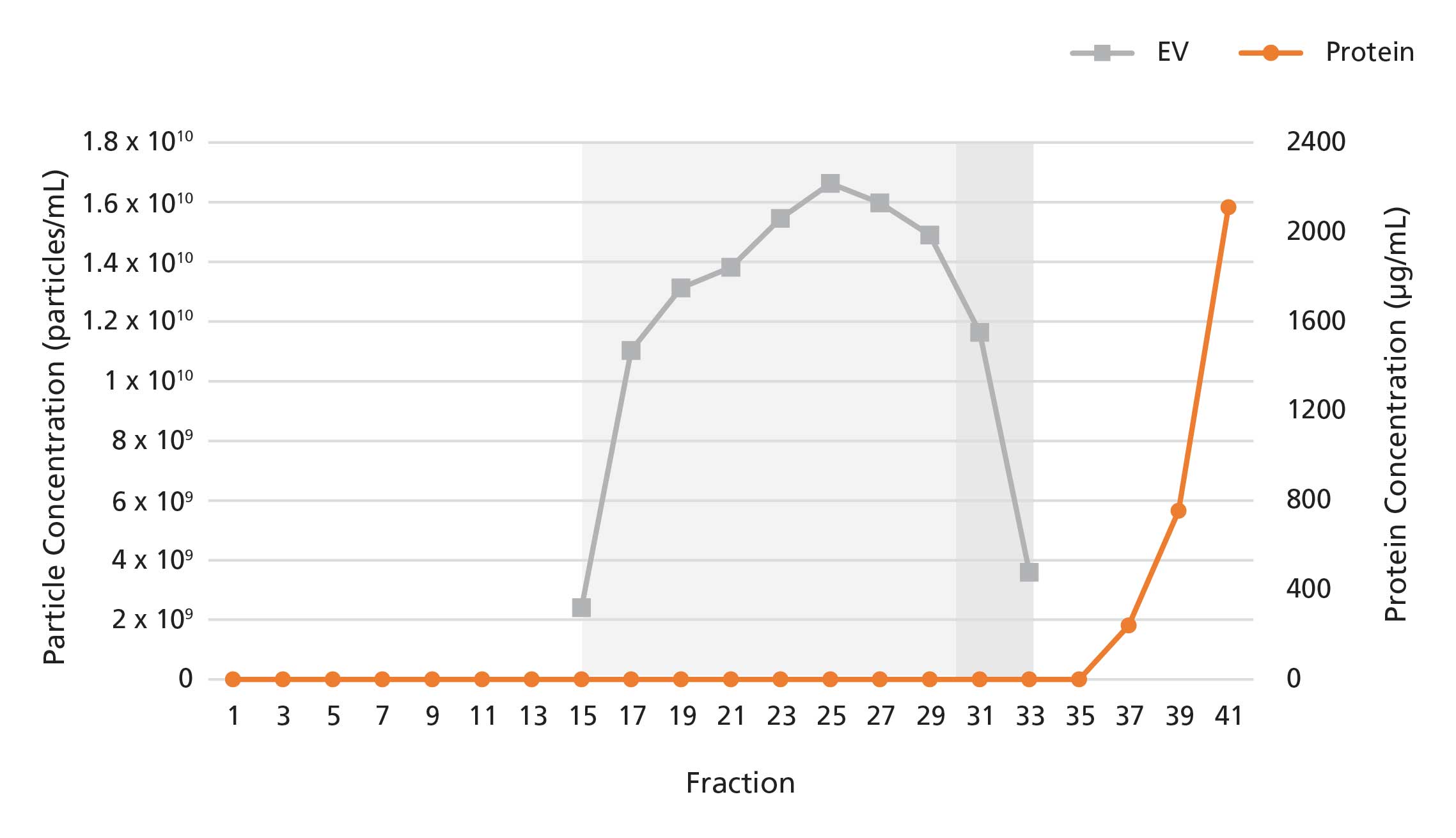
Figure 3. Isolation of Extracellular Vesicles from Conditioned Medium Using a 20 mL Extracellular Vesicle SEC Column
The 20 mL Extracellular Vesicle SEC Column was loaded with 20 mL of 10-fold concentrated serum-free medium conditioned by mesenchymal stromal cell (MSC) culture. 1 mL fractions were collected and analyzed for particle and protein content by nanoparticle tracking analysis and bicinchoninic acid assays, respectively. EVs were detected in fractions 15 - 30, while proteins were detected in fractions 35 onwards. Fractions 31-33, which are outside of the typical EV elution range, may still contain EVs with low protein contamination for serum-free media samples.
Find supporting information and directions for use in the Product Information Sheet or explore additional protocols below.
This product is designed for use in the following research area(s) as part of the highlighted workflow stage(s). Explore these workflows to learn more about the other products we offer to support each research area.
Thank you for your interest in IntestiCult™ Organoid Growth Medium (Human). Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration
| Species | Human, Mouse, Non-Human Primate, Other, Rat |
|---|

抗体试剂盒用于检测细胞外囊泡使用CD9, CD63和CD81标记

利用免疫磁阳性选择快速简便地分离人细胞外囊泡

利用免疫磁阳性选择快速简便地分离人细胞外囊泡

利用免疫磁阳性选择快速简便地分离人细胞外囊泡
扫描二维码或搜索微信号STEMCELLTech,即可关注我们的微信平台,第一时间接收丰富的技术资源和最新的活动信息。
如您有任何问题,欢迎发消息给STEMCELLTech微信公众平台,或与我们通过电话/邮件联系:400 885 9050 INFO.CN@STEMCELL.COM。

