产品号 #02032_C
用于人免疫球蛋白E的检测和测定
用于人免疫球蛋白E的检测和测定
人免疫球蛋白E (IgE)酶联免疫吸附测定试剂盒是专为定量检测和测量人的IgE在生物流体,如血清,血浆和细胞培养上清。IgE主要存在于肺、皮肤和粘膜中,是血清中最不常见的免疫球蛋白。IgE通过与Fcε受体I (Fcε ri)相互作用,刺激嗜酸性粒细胞、肥大细胞和嗜碱性粒细胞的脱颗粒,在过敏反应中发挥重要作用。
该检测基于夹心ELISA方法,将样品添加到酶联免疫吸附试验(ELISA)带材板上,该带材板上预先涂有捕获的免疫球蛋白特异性抗体。捕获的免疫球蛋白通过添加生物素化检测抗体检测,然后添加链亲和素-辣根过氧化物酶(SA-HRP),它与生物素化抗体结合。添加显色酶底物3,3 ‘,5,5 ’四甲基联苯胺(TMB)可得到着色产物,其强度与样品中免疫球蛋白的浓度成正比。免疫球蛋白的浓度是通过与平行分析的免疫球蛋白标准品的连续稀释度进行比较来确定的。
Subtype
Complete Kits
Cell Type
B Cells, Hybridomas
Species
Human
Area of Interest
Hybridoma Generation, Immunology
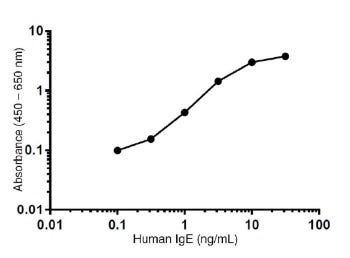
Representative Standard Curve
• Reportable Range: 0.1 - 32 ng/mL. This is the concentration range in which measurement of the analyte can be done with the highest precision, accuracy, and linearity.
• Sensitivity: The limit of detection of this assay is 0.069 ng/mL. This is the analyte concentration with absorbance two standard deviations higher than the zero standard.
• Accuracy: The analyte standard of this ELISA was calibrated against NIBSC international standard 75/502.
• Recovery: A mid-curve recovery of 80 - 86% was determined by spiking defined amounts of analyte standard into serum or plasma samples in repeated experiments.
• Precision: The intra-assay precision of this assay is 2.8% (CV). The inter-assay precision of this assay is 5.5% (CV).
Find supporting information and directions for use in the Product Information Sheet or explore additional protocols below.
This product is designed for use in the following research area(s) as part of the highlighted workflow stage(s). Explore these workflows to learn more about the other products we offer to support each research area.
Thank you for your interest in IntestiCult™ Organoid Growth Medium (Human). Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration
| Species | Human |
|---|

骨髓瘤和杂交瘤培养基(含血清)
次黄嘌呤胸腺嘧啶杂交瘤生长培养基(含血清)

用于分离单个核细胞的密度梯度离心液

免疫磁珠负选人B细胞
扫描二维码或搜索微信号STEMCELLTech,即可关注我们的微信平台,第一时间接收丰富的技术资源和最新的活动信息。
如您有任何问题,欢迎发消息给STEMCELLTech微信公众平台,或与我们通过电话/邮件联系:400 885 9050 INFO.CN@STEMCELL.COM。