产品号 #05925_C
包含从外周血中分离和扩增CD34+祖细胞及将其重编程为iPS细胞的工作流程
包含从外周血中分离和扩增CD34+祖细胞及将其重编程为iPS细胞的工作流程
CD34+祖细胞重编程试剂盒包含一套完整的工具和试剂,用于从人外周血样本中富集、扩增和重编程CD34+祖细胞。
亚型
专用培养基
细胞类型
造血干/祖细胞,多能干细胞
种属
人
应用
细胞培养,重编程
品牌
StemSpan,TeSR
研究领域
干细胞生物学
制剂类别
无血清
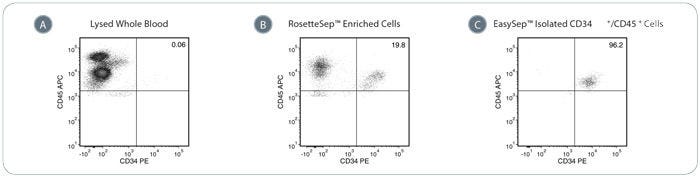
Figure 1. Typical Performance of the EasySep™ Complete Kit for Human Whole Blood CD34+ Cells
(A) In whole PB, the proportion of CD34+/CD45+ cells is typically very low (<1%). (B) In the RosetteSep™ enrichment step, unwanted cells are removed during density gradient centrifugation. This step enriches the population of CD34+/CD45+ cells (19.8%) in this example). (C) After positive selection of CD34+ cells using EasySep™, a highly pure population of CD34+/CD45+ cells (96.2% in this example) can be obtained. Values are reported as a percentage of viable CD45+ cells.
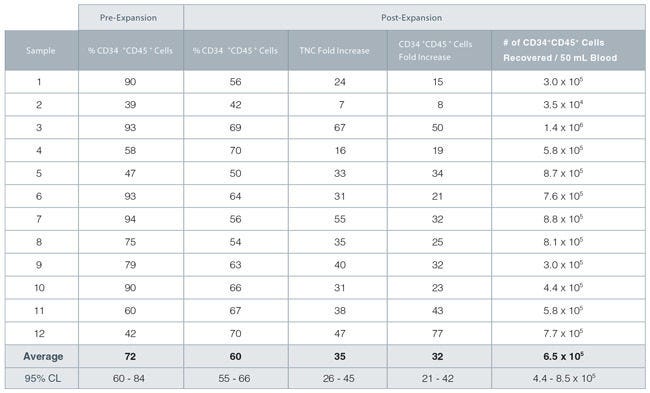
Figure 2. StemSpan™ SFEM II and CD34+ Expansion Supplement Support Expansion of CD34+ Cells
TNC: Total Nucleated Cell
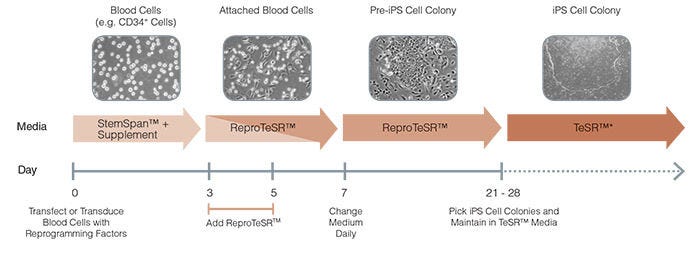
Figure 3. Schematic of ReproTeSR™ Reprogramming Timeline
ReproTeSR™ is used during the entire induction phase of reprogramming (day 3 to 21). On days 3 and 5, ReproTeSR™ is added to StemSpan™ growth media (in a fed-batch manner) to facilitate attachment of transfected cells. Attached cells are further cultured in ReproTeSR™ with daily full media changes until putative iPS cell colonies emerge (days 21-28). iPS cell colonies can then be isolated and propagated in TeSR™ media. (mTeSR™1, TeSR™2, TeSR™-E8™).
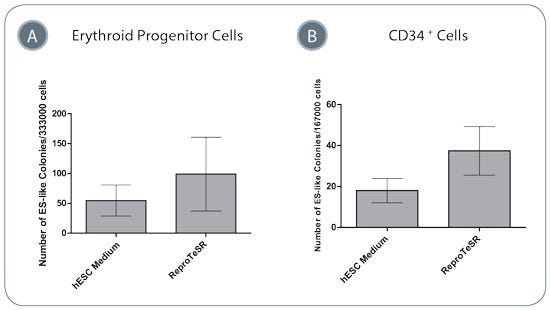
Figure 4. Blood Cell Reprogramming Efficiencies Are Higher in ReproTeSR™ Medium Compared to in hESC Medium
Efficiency of reprogramming (A) erythroid cells, or (B) CD34+ cells using episomal reprogramming vectors is higher in ReproTeSR™ medium compared to in KOSR-containing hESC medium. Data shown are mean +/- SEM, erythroid cells n=4, CD34+ cells n=5.
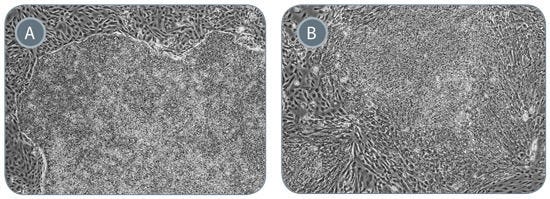
Figure 5. ReproTeSR™ Generates iPS Cell Colonies With Superior Colony Morphology
Representative images of iPS cell colonies generated from isolated CD34+ progenitor cells using (A) ReproTeSR™ and (B) hESC) medium. iPS cell colonies produced using ReproTeSR™ exhibit more defined borders, compact morphology and reduced differentiation compared with hESC medium. 200X magnification.
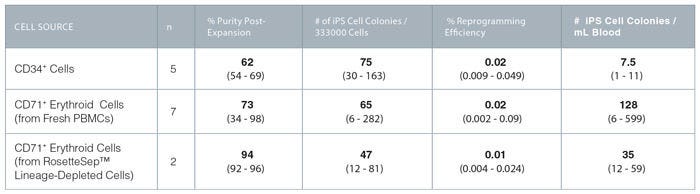
Figure 6. Reprogramming Efficiency of CD34+ and Erythroid Progenitor Cells With ReproTeSR™
Average values in bold (range).
Find supporting information and directions for use in the Product Information Sheet or explore additional protocols below.
This product is designed for use in the following research area(s) as part of the highlighted workflow stage(s). Explore these workflows to learn more about the other products we offer to support each research area.
Thank you for your interest in IntestiCult™ Organoid Growth Medium (Human). Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration
| Species | Human |
|---|---|
| Formulation Category | Serum-Free |
用于从外周血中分选和扩增红系祖细胞,并将其进一步重编程为诱导多能干细胞(iPS细胞)试剂盒
成分明确的无异源基质,支持人多能干细胞在无血清、无饲养层条件下的生长和分化。
扫描二维码或搜索微信号STEMCELLTech,即可关注我们的微信平台,第一时间接收丰富的技术资源和最新的活动信息。
如您有任何问题,欢迎发消息给STEMCELLTech微信公众平台,或与我们通过电话/邮件联系:400 885 9050 INFO.CN@STEMCELL.COM。